
- •Adenylyl cyclase
- •Cyclic AMP: the first second messenger
- •cAMP is formed from ATP
- •Adenylyl cyclase and its regulation
- •Structural organization of adenylyl cyclases
- •Regulation of adenylyl cyclase
- •Regulation by GTP binding proteins
- •Regulation by phosphorylation
- •Aluminium fluoride
- •Forskolin
- •Cholera and pertussis toxins and ADP ribosylation
- •ADP-ribosylation and deribosylation: a general mechanism of cell control
- •Phospholipase C
- •First hints of a signalling role for inositol phospholipids
- •The phospholipase family
- •Phospholipase C
- •The isoenzymes of PLC
- •Regulation of PLC
- •References
Signal Transduction
The human multidrugresistant P-glycoprotein, encoded by the MDR1 gene, is a member
of the family of ABC (ATP-binding cassette) transporters. Overexpression causes resistance of human cancers to chemotherapy.
CFTR and P-glycoproteins have the signature of
an ATP-driven transport protein, also known as pumps. This may explain why adenylyl cyclase has maintained an ATP binding site. Strangely
enough, CFTR behaves as a chloride ion channel (for reasons not understood), but P-glycoproteins behave as real pumps, even to the point of operating with a kind of piston-like mechanism with two cylinders.15
by parts of both the C1a and C2a subdomains, but probably in a location much closer to the catalytic centre. Although nucleotides, forskolin, and s can bind to residues on both C1a and C2a, it appears that -subunits potentiate the activity of AC2 by interacting only with the C2a subdomain. This causes a
conformational change that indirectly enables it to modulate the conformation of the C2a and so indirectly promotes optimal alignment at the catalytic site. The inhibitory actions of -subunits on AC1 must occur elsewhere since the sequences are not conserved in this region of the two subtypes.
The C1 and C2 domains, shorn of their extensive membrane attachments, are together sufficient to catalyse the conversion of ATP to cAMP.12,13 Although the activity is very low, the association between them is enhanced and
the rate of conversion is increased more than 100-fold by forskolin or the presence of s.14 These two activators are synergistic, the presence of the one enhancing the affinity of the other.
Although the M1 and M2 domains clearly anchor the enzyme to the membrane, one would imagine that one or two transmembrane spans would suffice for this purpose and it is reasonable to ask why these enzymes are endowed with 12. The answer probably lies in their evolutionary ancestry. It is certainly intriguing that the general membrane topology of the mammalian cyclases, particularly the two groups of six membrane-spanning segments, bears a strong resemblance to known pore-forming structures such as the cystic fibrosis transmembrane conductance regulator (CFTR) and the P- glycoproteins. However, no ion channel-like activity has been detected for any of the mammalian adenylyl cyclases, nor do they exhibit any sequence similarity with known channels.
Sequence similarities certainly extend to the guanylyl cyclases and to some non-mammalian forms of the enzyme expressed in lower organisms, such as the slime mould Dictyostelium. In general, a common ancestry can be inferred. More distantly, the yeast and bacterial (E. coli) enzymes are membraneattached, but are not true intrinsic membrane proteins. Sequence similarities between these and the mammalian cyclases have been hard to discern.
While Gs originally denoted a stimulatory activity and Gi denoted inhibition with respect to cyclase, these G proteins also regulate many other systems.
Regulation of adenylyl cyclase
With the emergence of the metazoans, the regulation of cyclase came under the control of heterotrimeric G proteins. In the few systems in which it has been possible to examine stoichiometry of the components leading to the synthesis of cAMP, it appears that s is present in enormous excess over both its controlling receptors and the cyclase. Thus, for rat ventricular myocytes, the level of effector enzyme is three times that of the receptor, while the level of Gs is in great excess over either (some 200-fold).16 Because the level of cyclase is so low, the signal amplification that occurs with the activation of the
136

Effector enzymes coupled to GTP binding proteins
G protein is not maintained. This sets an upper limit to the rate of synthesis of cAMP.17
All isoforms of mammalian adenylyl cyclase are activated by the -subunit of Gs and all are inhibited by the so-called P-site inhibitors (analogues of adenosine such as 2 -deoxy-3 -AMP). Beyond this, their responses to the
many and various inhibitory ligands vary widely. With the exception of AC9, all are activated by forskolin which acts in synergy with s. They are influenced, both positively and negatively, by the - and -subunits of other G proteins, by Ca2 , and through phosphorylation by PKA and PKC (see Table 5.1). It appears that their evolutionary development has determined their specialized sensitivities towards the various regulatory influences.
Broadly, they can be grouped in three main classes:
•
•
•
AC2, AC4, and AC7, which are activated synergistically by s- and - subunits
AC5 and AC6, which are inhibited by i and Ca2
AC1 and AC8, which are activated synergistically by s together with Ca2 - calmodulin (AC3 has been included in this class, but its activation by Ca2 in vivo is uncertain. It is inhibited by Ca2 -calmodulin kinase II).
So far, there is limited information concerning the precise roles of these individual isoforms.18 A lack of good antibodies has prevented attempts to determine their subcellular localization and only for AC1, AC3, and AC8 do we have information from knock-outs. This is a tricky field in which to venture, as illustrated by RNAi knock-down experiments that have revealed a remarkable degree of interdependence in the expression of G-protein
subunits and AC isoforms, so that elimination of one component may cause compensatory expression or increased activity of others. For example, knockdown of G 1 and G 2 increased the expression of G 4. It also increased the expression of some AC isoforms and reduced that of G i. The overall result was an unexpected increase rather than a decrease in cyclase activity.19 It is noteworthy that no human disease has so far been linked to mutations in the adenylyl cyclases.
Regulation by GTP binding proteins
Since the Gi proteins are generally expressed at a level greatly exceeding that of Gs, stimulation of receptors linked to Gi can generate considerable quantities of activated -subunits. It has been proposed that this excess of -subunits should be able to sequester free s-subunits, opposing their ability to activate cyclase:
βγ αs → αsβγ
137

138
Table 5.1 Regulatory signals
Isoform |
Distribution |
|
i |
0 |
z |
PKA |
PKC |
Forskolin |
Ca2 – |
Ca2 (CaM |
|
|
|
|
|
|
|
|
|
CaM* |
indep) |
|
|
|
|
|
|
|
|
|
|
|
AC2 |
brain, lung |
↑ |
n/e |
n/e |
|
|
↑ |
↑ |
n/e |
|
|
|
|
|
|
|
|
|
|
|
|
AC4 |
widely distributed: |
↑ |
|
|
|
|
↑ |
↑ |
|
|
|
|
|
|
|
|
|
|
|
|
|
AC7 |
widely distributed |
↑ |
|
|
|
|
↑ |
↑ |
n/e |
|
|
|
|
|
|
|
|
|
|
|
|
AC5 |
heart brain |
n/e |
↓ |
n/e |
↓ |
↓ |
↑ |
↑ |
n/e |
↓ |
|
|
|
|
|
|
|
|
|
|
|
AC6 |
heart, brain other |
n/e |
↓ |
n/e |
|
↓ |
↓ |
↑ |
n/e |
↓ |
|
tissues |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AC8 |
neural tissue |
↓ |
|
|
|
|
n/e |
↑ |
↑ |
|
|
|
|
|
|
|
|
|
|
|
|
AC1 |
neural tissue |
↓ |
↓ |
↓ |
↓ |
|
|
↑ |
↑ |
|
|
|
|
|
|
|
|
|
|
|
|
AC3 |
predominantly |
n/e |
|
|
|
|
|
↑ |
↑ |
|
|
olfactory |
|
|
|
|
|
|
|
|
|
Transduction Signal
CaM, calmodulin; n/e, no effect.
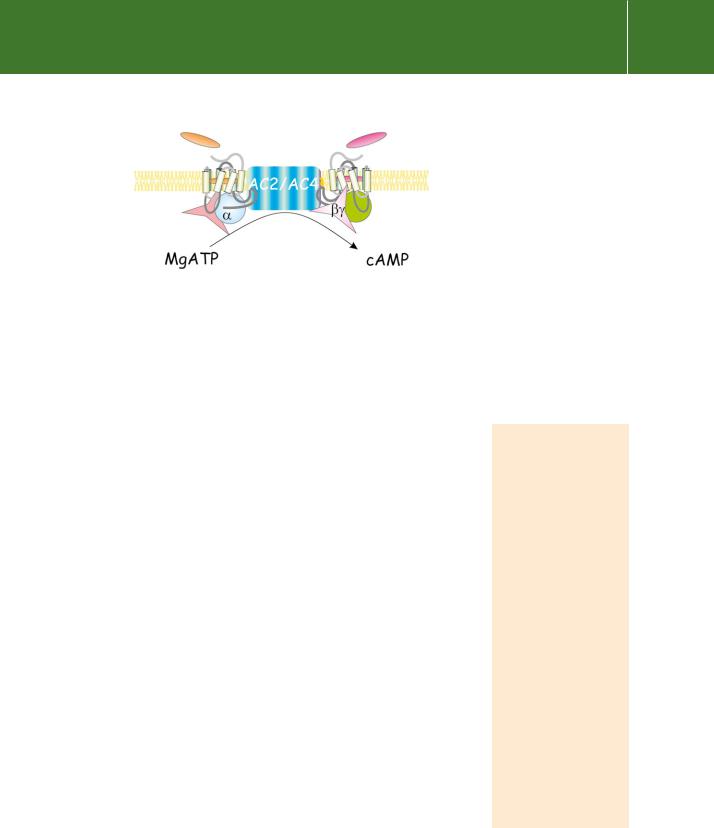
Effector enzymes coupled to GTP binding proteins
For this to operate as an inhibitory mechanism, it would be necessary for the subunits to exist as independent entities, but as we have argued, it is unlikely that s ever separates from (see page 111). However, AC1 and AC8 are inhibited by -subunits, but here the effect is primarily directed through interaction with the enzyme, not by sequestration of -subunits. On the other hand, -subunits synergize with s to activate AC2 and AC4 cyclases. As one might expect, there is little if any effect of the -subunits in the absence
of s. The level of -subunits required is much greater than could possibly be derived from Gs alone, but it is fully commensurate with the amounts that could be generated through the activation of Gi or Go. This implies that two sets of hormone receptors must be activated simultaneously in order to ensure maximal stimulation (Figure 5.4). It is possible that the dimeric structure of many receptors (including the -adrenergic receptor; see page 69) enables them to operate as a coincidence detectors, inducing a powerful
response when the two signals are generated, but only a weak or insignificant response to unsynchronized signals. This is the likely explanation for the costimulatory effect of noradrenaline and adenosine on the cyclase activity in brain cortical slices,20 mentioned in Chapter 3 (see page 70). The presence of particular subtypes of adenylyl cyclase could then determine distinct patterns of response due to the signals from different classes of receptors. Figure 5.5 presents several possible schemes that explain how the converging pathways might interact to cause activation or inhibition. This could be of particular importance in synapses, allowing them to coordinate their responses to incoming stimuli. One of the signals would be transmitted by s, the other would be coupled to the more prolific Gi or Go that would generate the required amounts of -subunits.
So far, with the exception of 1 1 which is located exclusively in the retina, it has been hard to establish any specific functional correlates of the diverse-pairs. Combinations of 1 or 2 with any of the four -isoforms 2, 3,
5, or 7, appear to inhibit AC1 and to activate AC2 with equal effect. They also activate the -isoforms of phospholipase C (see below). This lack of specificity is surprising and could be an artefact, arising from the high concentrations of -subunits which must be provided in reconstitution experiments.
Fig 5.4 Adenylyl cyclase as a coincidence detector, integrating the signals from two G proteins.
Alternatively, the lack of specificity could be real. Though never demonstrated, - subunits might be subject to a transient covalent modification following interaction
with activated receptors (ADP-ribosylation: see below). This could allowdimers to impose their effects on downstream effectors at much
lower concentrations.21 Modified -subunits could then, in principle, remain active beyond the time that the -subunits have reverted to their GDP state. It might also allow for specificity among the different pairs, depending on their association with particular-subunits and particular classes of receptors.
139
Signal Transduction
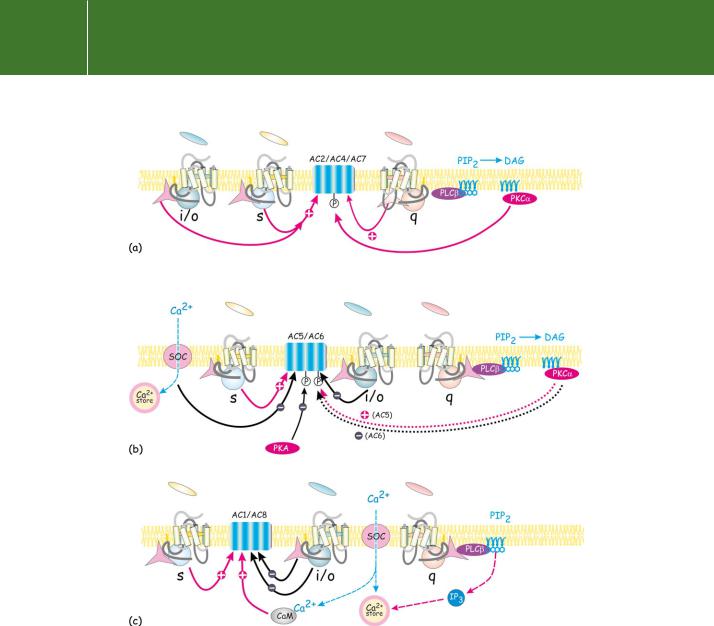
Fig 5.5 Adenylyl cyclase isoforms: patterns of activation and inhibition. The adenylyl cyclases are subject to regulation by multiple signals. These may cooperate or oppose each other. (a) In some instances the activation of AC2, 4, and 7 by s together with -subunits occurs with a high level of synergy. Activation is only achieved when two distinct classes of receptor are activated simultaneously. On the other hand, the phosphorylation of these same enzymes by PKC is likely to be of longer duration, so that the enzyme is set up in readiness for stimulation by s. (b) AC5 and AC6 are subject to indirect feedback inhibition by phosphorylation of the enzyme by PKA. They are also inhibited by Ca2 and by receptors linked through the Gi proteins. (c) AC1 and AC8 are activated by Ca2 -calmodulin and inhibited by -subunits. (SOC indicates a store-operated Ca2 channel.)
Clearly, there are several routes to the activation of adenylyl cyclase and the elevation of cAMP, but equally important are those influences which inhibit its activity. There are many receptors (for example, the 2-adrenergic receptors) that are linked to the group of G proteins that have i-subunits. These inhibit AC5 and AC6.
140
