
- •Adenylyl cyclase
- •Cyclic AMP: the first second messenger
- •cAMP is formed from ATP
- •Adenylyl cyclase and its regulation
- •Structural organization of adenylyl cyclases
- •Regulation of adenylyl cyclase
- •Regulation by GTP binding proteins
- •Regulation by phosphorylation
- •Aluminium fluoride
- •Forskolin
- •Cholera and pertussis toxins and ADP ribosylation
- •ADP-ribosylation and deribosylation: a general mechanism of cell control
- •Phospholipase C
- •First hints of a signalling role for inositol phospholipids
- •The phospholipase family
- •Phospholipase C
- •The isoenzymes of PLC
- •Regulation of PLC
- •References

Effector enzymes coupled to GTP binding proteins
Regulation by Ca2
Ca2 is also an important intracellular second messenger and an important regulator of adenylyl cyclases.22 The signalling mechanisms that cause an elevation of intracellular Ca2 are considered in detail in Chapters 7 and 8. Briefly, cytosol [Ca2 ] increases when the ion enters cells through plasma membrane channels, such as voltage-activated Ca2 channels. Alternatively, it may be released into the cytosol from internal stores when phospholipase C (PLC) is activated by a receptor mechanism. For example, receptors that are coupled to Gq regulate PLC . In association with the protein calmodulin (see Chapter 8), Ca2 increases the activity of AC1 and AC8. A binding site for calmodulin is present on the C1b sub-domain of AC1 and there is also a candidate site on AC8. In contrast, AC5 and AC6 are both inhibited by Ca2 in a calmodulin-independent manner. The predominantly olfactory AC3 is also Ca2 -sensitive, but the outcome is unclear. It is activated by Ca2 in the
presence of calmodulin in vitro, but it lacks an identifiable calmodulin binding site and it is inhibited by Ca2 in human embryonic kidney cells (HEK293).
In non-excitable cells, the increase in concentration achieved following activation of Ca2 -mobilizing receptors is not high enough to have more than a limited effect on the Ca2 -sensitive adenylyl cyclases. Rather, it is Ca2 entering the cell through plasma membrane channels to replenish depleted intracellular stores that is responsible; (store-operated Ca2 entry or SOCE
is described in Chapter 7). In excitable cells, Ca2 entry through voltageactivated channels is also effective. It thus appears that location of the cyclases close to the high levels of Ca2 entering through open channels is important.22
In general, the distribution over the plasma membrane of channels, receptors, mediators, and effectors is not uniform. Some occupy or may be recruited
to dynamic plasma membrane microdomains rich in cholesterol and glycosphingolipids, known as lipid rafts (see page 522). The Ca2 -regulated ACs (AC1, 3, 5, 6, 8) are concentrated in raft regions, where they coreside with a set of regulators and effectors, such as G-protein-coupled receptors, G proteins, putative SOCE components, and recruited phosphodiesterases. In contrast, the Ca2 -insensitive AC2, AC4, and AC7 are excluded from rafts. The compartmentalization of the Ca2 -sensitive ACs with relevant signalling proteins and the phosphodiesterases (PDEs) that break down cAMP provides a machinery that is able to create localized pockets of briefly elevated cAMP.
The ability of the Ca2 -dependent ACs to be activated by both Gs and Ca2 provides another mechanism of coincidence detection.
Regulation by phosphorylation
Although one might expect that phosphorylation by PKA (activated by cAMP) might cause feedback inhibition, this has only been detected for AC5 and AC6. On the other hand, phosphorylation by PKC enhances the activity of AC2,
141
Signal Transduction
More recent examination of the crystal structure of AlF4 has revealed that in association with GDP, the complex is a square-planar entity.26 Rather than behaving strictly as an appendage, forming a GTP lookalike, it resembles the transition state in the GTPase reaction. It is widely accepted that the interaction with the [AlF4 ] is characteristic
only of the heterotrimeric G proteins. However, it has become apparent that monomeric GTPases such as Ras also interact with AlF4 when they are associated with GTPaseactivating proteins
such as p120GAP and neurofibromin.29,30
A member of the mint family, Coleus forskohlii grows wild on the mountain slopes of Nepal, India, and Thailand. The tuberous roots have long been used as a spice and by practitioners of Ayurvedic medicine in the treatment of many diverse conditions related to heart disease, spasmodic pain,
painful micturition, and convulsions.
AC4, AC7, and AC5 (AC6 is inhibited). This is a consequence of the activation of receptors linked through G proteins of the Gq/11 family to the activation of phospholipase , generating diacylglycerol, the activator of PKC. The precise timing of signals transmitted through s is likely to be less critical because
a phosphorylated state of the enzyme is expected to persist for longer than other more transient influences, such as those activated by - or -subunits, or elevated Ca2 . Rather than requiring a coincidence of signals, phosphorylation may set the enzyme up in readiness for transient stimulation by s.
Aluminium fluoride
Adenylyl cyclase can be activated experimentally by manipulations that bypass the receptors. A number of substances cause activation through interaction with Gs, others with the cyclase itself. An example is the fluoride ion.23,24 Here is yet another instance of a development in which the emergence of highly purified reagents led first to the loss of biological activity and subsequently to the identification of its real nature. With better chemicals, well-dialysed tissue extracts, the use of laboratory plastic instead of glassware, and the availability of high-quality water, it became harder to stimulate cyclase with fluoride. The real activator, it turns out, is not the fluoride ion
but a complex of aluminium and fluoride ([AlF4 ]).25 Aluminium is present as a contaminant of solutions that have been in contact with glass and of commercially produced nucleotides. It has been thought that the [AlF4 ] complex, resembling a phosphate group in its shape and charge distribution, becomes trapped adjacent to GDP in the nucleotide binding pocket of-subunits in the site that would be occupied by the -phosphate of GTP.
At higher concentrations, rather than activating, fluoride acts to suppress the formation of cAMP. This finding gave the first inkling of the G proteins that signal the inhibition of cyclase activity.27,28
Forskolin
Another activator that has already been mentioned is forskolin (Figure 5.6), a diterpene isolated from the roots of Coleus forskohlii. Unlike aluminium fluoride, this interacts directly with the cyclase enzyme (see Figure 5.3).
Physiologically, forskolin has a positive inotropic action: it increases heart rate and lowers blood pressure. It also exhibits non-specific spasmolytic activity on gastrointestinal smooth muscle. At large doses it has a depressant action on the central nervous system. Some of these phenomena could arise as a consequence of the direct action of forskolin on adenylyl cyclase, bypassing all upstream influences including receptors and GTP-binding proteins. Alternatively, they could be due to interactions with other intrinsic membrane proteins of related structure, including glucose transporters, voltage-gated K channels, and the P-glycoproteins that confer multidrug resistance. The existence of natural products that react potently and specifically as regulators
142

Effector enzymes coupled to GTP binding proteins
Fig 5.6 Forskolin.
of enzyme activity, begs questions relating to their endogenous physiological counterparts. So far however, no endogenous agent having the activity of forskolin has been identified.31
Cholera and pertussis toxins and ADP ribosylation
The accumulation of cAMP is enormously promoted in tissues that have been treated with cholera or pertussis toxins (respectively the exotoxins of Vibrio cholerae and Bordetella pertussis). Neither of these toxins has its
effect directly on the cyclase enzyme. Instead, cholera toxin acts to prolong the activated state of the s. This situation arises because it inhibits the GTPase activity so that the bound GTP remains intact. The system becomes persistently activated. It is widely (but possibly mistakenly) accepted that the watery diarrhoea of cholera, the consequence of increased ion flux through the chloride channels of the intestinal epithelium, results from the elevated concentration of cAMP due to the maintained activation of adenylyl cyclase.
Pertussis toxin also has the effect of elevating the level of cAMP, but its mechanism of action is quite different. Intoxication by pertussis toxin plays a role in the aetiology of whooping cough.33 The affected cells include the-cells of pancreatic islets (hence its former name, islet activating protein), lymphocytes, leukocytes, cardiac cells, and others that cause paroxysms and neurological disturbance. The effects on these cells are irreversible, so that the restoration of function depends on their replacement. Unlike cholera toxin, which acts to inhibit GTPase activity, pertussis opposes the signal transmission by inhibitory receptors communicating with the Gi proteins.
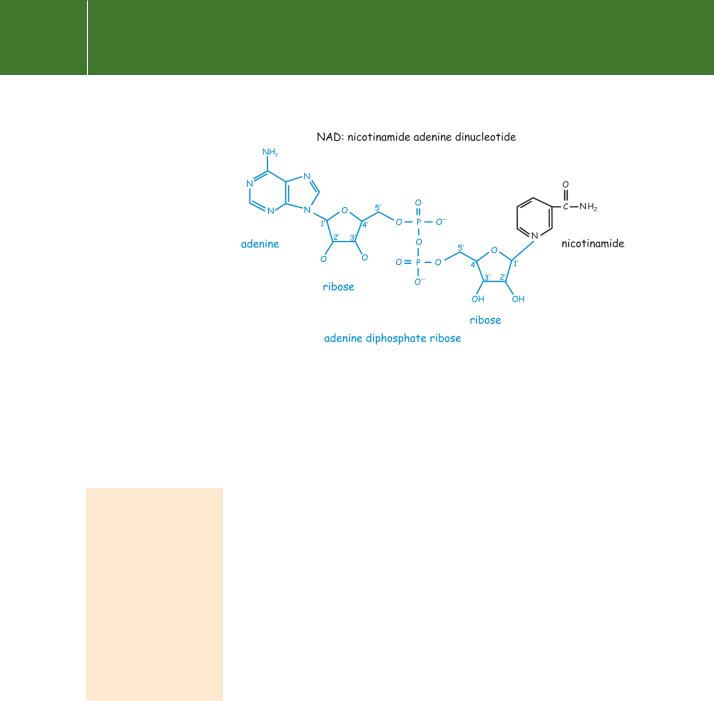
Both cholera and toxin are enzymes, modifying their target proteins (substrates) by covalent attachment of the ADP-ribosyl moiety of NAD (Figure 5.7).34,35 However, in keeping with their distinct mechanisms of action, their site specificities are quite different. Cholera toxin transfers ADP-ribose to s, forming a covalent attachment with an arginine residue (R201) situated in the close vicinity of the -phosphate of the bound GTP (see Table 4.5, page 103). This modification prevents the GTPase activity and so causes the -subunit to remain
The mechanism of cholera-induced diarrhoea is certainly more complicated than this. Indeed, although the level of cAMP is undoubtedly elevated, it may not even involve cAMP at all. Introduction of dibutyryl cAMP, a cell-permeant analogue which mimics the effect
of the natural compound, is without effect on
the rate of water flux through isolated pieces of intestinal tissue. Similarly, theophylline, that inhibits phosphodiesterase and so allows the accumulation of cAMP, is also without effect. Alternative candidates are the prostaglandins,32 products of the cyclooxygenase pathway of arachidonate metabolism. Arachidonate is released from membrane phospholipids by the action of phospholipase A2.
Similar to pertussis and cholera toxin, diphtheria toxin, from Corynebacterium diphtheriae, is also an
ADP-ribosylating enzyme. It acts on the elongation factor EF2. Unlike these other toxins it gains entry to cells by a mechanism involving endocytosis. One molecule of the toxin is sufficient to block the entire protein synthesizing machinery of a cell.
143
Signal Transduction
Note that in addition to the ADP-ribosylating toxins, both Vibrio cholerae and Bordetella pertussis synthesize and release many other cell-penetrating toxins. Interestingly, one of the
major virulence factors of
B. pertussis is a secreted form of adenylate cyclase which becomes activated in contact with cellular calmodulin.
Fig 5.7 ADP-ribose.
persistently activated (reduction of kcat: see Equation 4.1 on page 85). Pertussis toxin transfers ADP-ribose to i at a cysteine situated just four residues from its C-terminus. ADP-ribosylation by pertussis toxin has the effect of interrupting the communication between the receptor and Gi, freezing it in its GDP-bound state. In this way it prevents the negative signal from inhibitory receptors. Either way, the consequence is persistent activation of adenylyl cyclase and elevated levels of cAMP. With the exception of z, all members of the Gi/Go group of -subunits (Figure 5.7) serve as substrates for pertussis toxin. In most cells and tissues, the-subunit of Gs is the unique substrate for cholera toxin.
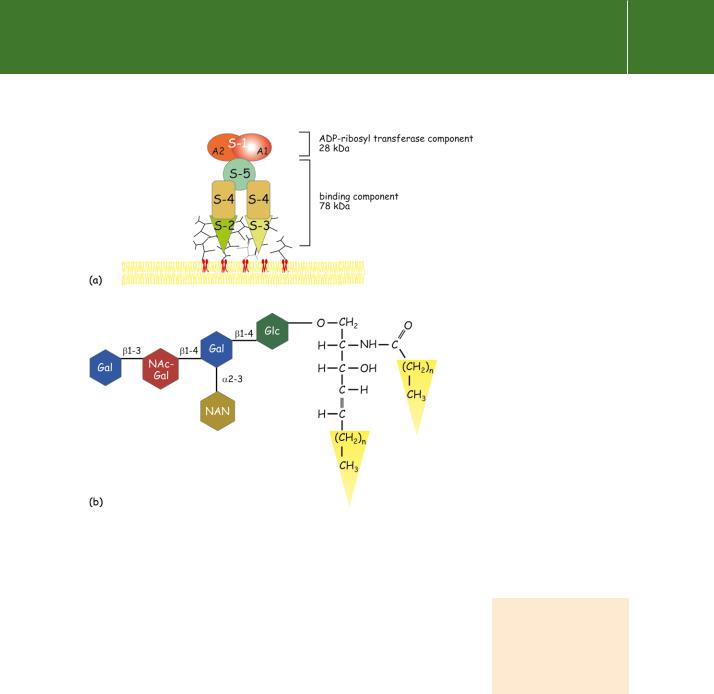
ADP-ribosylating enzymes such as cholera and pertussis toxins are complex structures. They bind to gangliosides that are ubiquitously expressed on the external surface of animal cells. Pertussis toxin is composed of five noncovalently linked subunits which are organized around a single catalytic subunit S1 (Figure 5.8). Two subunits (S2 and S3) bind with high affinity (KD0.6 mol l 1) to the monosialoganglioside Gd1a. As a result, hydrophobic domains are exposed on the single S1 subunit, which is thus enabled to penetrate the cell. Here, the reducing environment in the cytosol causes its separation into two components, A1 and A2. The A1 subunit catalyses the ADP-ribosylation reaction:
NAD protein → ADP-ribosyl-protein nicotinamide H
Like pertussis toxin, cholera toxin is composed of five non-covalently-linked subunits which are arranged in a ring-like pentameric formation surrounding a single catalytic-subunit. The B-subunits bind with high affinity (KD 1 nmol l 1) to the monosialoganglioside GM1, ubiquitously expressed on the surface
of all animal cells. (Cholera toxin also induces ADP-ribosylation of GTPases in plant cells.36,37) On gaining entry, the A1 subunit catalyses ADP-ribosylation.
144
Effector enzymes coupled to GTP binding proteins
When this reaction was first carried out in the presence of radioactively labelled NAD , the label was found to be transferred to a single membranebound protein, which, when isolated, proved to be the nucleotide-binding component ( -subunit) of Gs.34,38–40
As reagents, cholera and pertussis toxins have been instrumental in assigning particular receptors to particular G proteins in signalling pathways. The substrate arginine (R201) which is modified by cholera toxin is a crucial component of the GTPase catalytic site of s. Mutants generated by substituting R201, hydrolyse GTP at a greatly reduced rate (reduced kcat) and are in consequence persistently activated. Acquired (somatic) mutations at this site sometimes occur in non-metastatic endocrine tumours (adenomas). In spite of being non-invasive and restricted to a single clone of cells, adenomas have many dire consequences.
As an example, the physiological stimulus to the secretion of growth hormone from pituitary somatotrophs is provided by pulses of groth hormone relasing hormone (GHRH) released from the hypothalamus, especially during periods of deep sleep. This provides both the signal to secrete growth hormone and the trophic stimulus by which these cells maintain their normal number and
Fig 5.8 Pertussis toxin structure.
(a)The subunit organization of pertussis toxin. The S2 and S3 subunits bind to the exposed sugar residues
of glycolipids (gangliosides) in the plasma membrane. The S1 subunit penetrates the cell and, in the reducing environment, dissociates. The A1 component catalyses the cleavage
of NAD and effects the transfer of the ADP-ribosyl group to the cysteine residue at the C-terminus of i.
(b)Structure of ganglioside GM1. Gal, galactose; NAc, N-acetyl-; Glc, glucose; NAN, N-acetylneuraminic acid.
The word somatic refers to any cell of a multicellular organism that does not contribute to the production of gametes.
145
