
- •Adenylyl cyclase
- •Cyclic AMP: the first second messenger
- •cAMP is formed from ATP
- •Adenylyl cyclase and its regulation
- •Structural organization of adenylyl cyclases
- •Regulation of adenylyl cyclase
- •Regulation by GTP binding proteins
- •Regulation by phosphorylation
- •Aluminium fluoride
- •Forskolin
- •Cholera and pertussis toxins and ADP ribosylation
- •ADP-ribosylation and deribosylation: a general mechanism of cell control
- •Phospholipase C
- •First hints of a signalling role for inositol phospholipids
- •The phospholipase family
- •Phospholipase C
- •The isoenzymes of PLC
- •Regulation of PLC
- •References
Signal Transduction
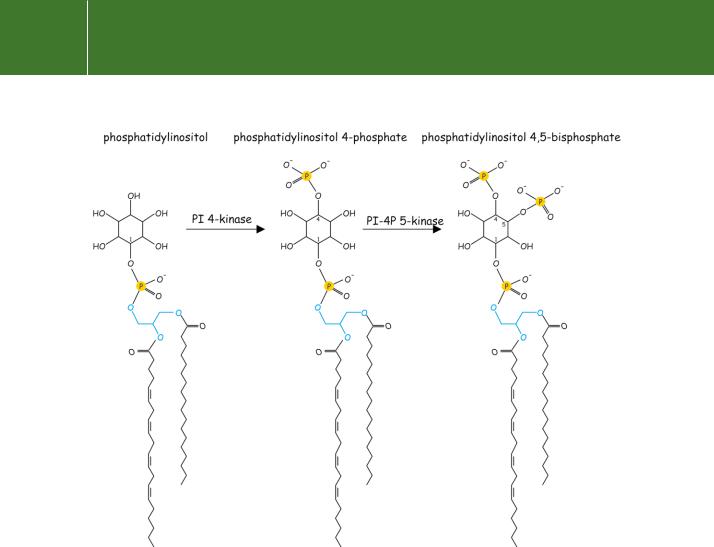
Fig 5.9 Phosphatidylinositol (PI) and its derivatives PI(4)P and PI(4,5)P2
Phospholipase C
The process by which IP3 is formed in response to agonist stimulation requires the activation of a plasma membrane-associated enzyme of the phospholipase C (PLC) family. Its substrate, PI(4,5)P2, is derived from
phosphatidylinositol through the action of lipid kinases (see Figure 5.9). In addition to the PLCs, PI(4,5)P2 is also the substrate of the phosphoinositide 3-kinases that cause further phosphorylation, generating the lipid second messenger PI(3,4,5)P3 (see Figure 18.1, page 546).
The isoenzymes of PLC
Eleven mammalian isoforms of PLC have been purified and cloned. These fall into four main classes: , , , and the recently discovered .50,51 Examination of the amino acid sequence reveals that all of the PLCs possess multiple structural domains. The isoform is the simplest, containing in addition to
148
Effector enzymes coupled to GTP binding proteins
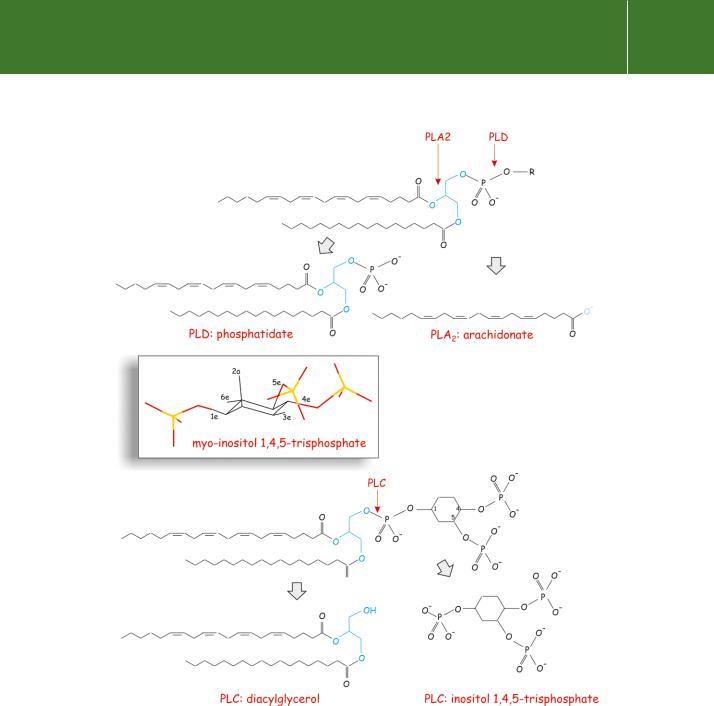
Fig 5.10 Phospholipases cleave specific phospholipid ester bonds. Top: arachidonate (together with a lysophospholipid) is the product of PLA2. Phosphatidate (and choline) are the products of PLD (the main substrate is phosphatidylcholine). The biological form of inositol (inset), based on cyclohexane, is myo -inositol, in which the hydroxy substitutions at positions 1, 3, 4, 5, and 6 are all equatorial (e), while the 2-position is substituted axially
(a). Bottom: the structures of phosphatidylinositol 4,5-bisphosphate (specifically, 1-stearoyl, 2-arachidonyl PI(4,5)P2) and its breakdown products produced by PLC: inositol trisphosphate and diacylglycerol. The glycerol nucleus is blue.
149

Signal Transduction
The status of a possibleisoform is uncertain since the cDNA originally associated with this enzyme was later found to code for a protein disulfide isomerase. Isoenzymes also exist within each class, as PLC 1–4, PLC 1–2, PLC 1–4.
the catalytic domain only PH and C2 domains (see Chapter 24) and a stretch containing EF-hand motifs (Chapters 8 and 24). All forms of PLC possess a C2 domain in the vicinity of the C-terminus. The catalytic centre, split into two separated subdomains, X and Y, possesses high sequence similarity. In the case of PLC , the sequence intervening between the X and Y domains extends to more than 500 residues and encompasses two SH2 domains and
a single SH3 domain (Figure 5.11, see also Table 24.1, page 769). In spite of their considerable separation in the primary sequence, in the folded structure the X and Y segments come together forming two halves of a single structural unit which forms the catalytic centre. The isoform, lacking a PH domain, contains two unique domains that allow it to bind and activate Ras.
PLC : a prototype
The isoforms of PLC are likely to be ancestral to the other animal PLC isoenzymes. They are of lower molecular weight and possess no domains that are not present in one or more members of the other classes. Some organisms such as the yeasts, Dictyostelium, and flowering plants contain only the forms. It is has been speculated that PLC emerged initially as a Ca2 -dependent generator of DAG, the activator of PKC.52 In this case, the water-soluble by-product, IP3, would have had no role to play in signalling. Later in evolution, as it too became a second messenger regulating Ca2 , more complex forms of PLC ( and ) emerged that are activated by receptors.
The relevance of the domains that are common to all the known isoforms is unclear. Although EF-hand motifs may bind Ca2 (Chapter 8), the N-terminal EF-hand-containing structure does not appear to be the site through which regulation by Ca2 is exerted. However, it is certainly of importance, possibly in a structural context, since the activity of the enzyme is abolished when about half of this domain is deleted. X-ray diffraction of PLC cocrystallized with IP3, which mimics the head group of PI(4,5)P2, has revealed that the regulating Ca2 ions are bound at the active site of the enzyme and also to the IP3 molecule.53 It is likely that the PH domain tethers the enzyme to the membrane by high affinity attachment to PI(4,5)P2. The C2 domain may then reinforce this attachment in a Ca2 -dependent manner. In this way it may optimize the orientation so that the active site can access its substrate in the membrane (Figure 5.12).
While the and isoforms of PLC are certainly more central to the regulation of cellular activity by receptors, a basis for the understanding of their regulation is provided by knowledge of the simpler isoform. It may also offer clues about the operation of other signal transduction enzymes (such as PKC (Chapter 9), cytosolic PLA2, and PI 3-kinase (Chapter 18) that use similar structural modules for reversible association with membranes.
150
