
- •Protein tyrosine phosphatases
- •Cytosolic PTPs
- •Transmembrane receptor-like PTPs
- •Tyrosine specificity and catalytic mechanism
- •PTPs in signal transduction
- •PTP1B, diabetes, and obesity
- •PTP1B as a possible therapeutic target for the treatment of type 2 diabetes and obesity
- •Redox regulation of PTP1B: reactive oxygen species as second messengers
- •Regulation of SHP-1 and -2
- •SHP-1, JAKs, and STAT5
- •SHP-2 and the Ras–MAP kinase pathway
- •Insight through the Noonan syndrome
- •Density enhanced PTP (DEP1)
- •CD45 and the regulation of immune cell function
- •Regulating receptor PTPs
- •Dual specificity phosphatases
- •Regulation of MAP kinases by dual-specificity protein phosphatases (DS-MKP)
- •Physiological role of the dual-specificity MAP kinase phosphatases
- •Dual-specificity phosphatases in development
- •PTEN, a dual-specificity phosphatase for phosphatidyl inositol lipids
- •Serine/threonine phosphatases
- •Classification of the serine/threonine phosphatases
- •Regulation of PPPs
- •Phosphorylation of the catalytic subunits
- •Regulation by intramolecular domain interaction
- •Regulatory subunits of PP1
- •Inhibitors of PP1, PP2A, PP4, and PP5
- •PP1 in the regulation of glycogen metabolism
- •Regulation of glycogen metabolism: muscle
- •Regulation of glycogen metabolism: liver
- •PP2B (calcineurin)
- •Dephosphorylation of NFAT: immunophilins show the way
- •References

Chapter 21
Protein Dephosphorylation
and Protein Phosphorylation
Protein phosphorylation serves multiple roles in the regulation of cell function. But this is only half the story. If the transfer of phosphate
groups to proteins is to serve as a precise and sensitive signalling mechanism, then necessarily it must operate against a low background. Dephosphorylation is therefore as important as phosphorylation, and it follows that the phosphoprotein phosphatases are integral components of the signalling systems operated by the protein kinases.1 In a number of cases dephosphorylation serves as a true reset button, bringing proteins back to their inactive state.
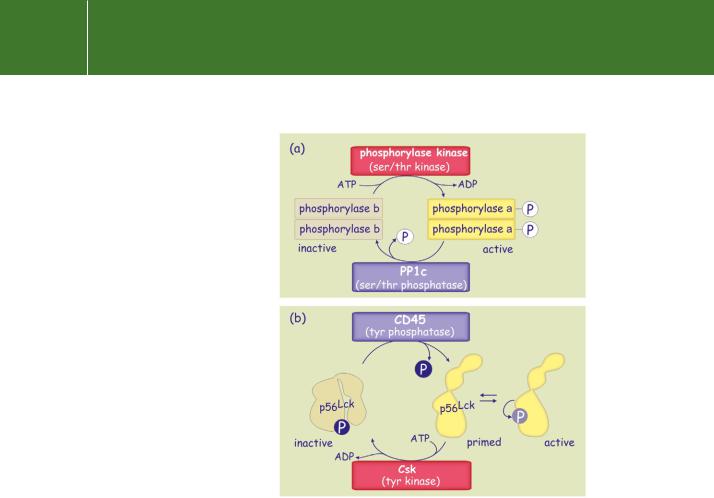
A good example of this is the role of the serine/threonine phosphatase PP1c which dephosphorylates glycogen phosphorylase a, thereby terminating the breakdown of glycogen. There are other proteins (for example, glycogen synthase, GSK3 , Src, Lck, c-Jun, and NFAT) that are inactive when phosphorylated, only becoming active as a consequence of dephosphorylation (Figure 21.1) In the case of Lck, a tyrosine protein kinase,
activation of the enzyme requires dephosphorylation of a C-terminal tyrosine
641
Signal Transduction
FIG 21.1 Phosphatases in the generation of signals for both deactivation and activation.
(a) A protein phosphatase as a reset button. The serine phosphatase PP1c dephosphorylates and deactivates phosphorylase a. (b) A protein phosphatase as activator. CD45, a receptor-like tyrosine protein phosphatase, primes p56Lck for activation.
residue and then phosphorylation of another tyrosine in the activation segment, through an autophosphorylation mechanism (see pages 532 and 659). To become fully active, the transcription factor c-Jun undergoes dephosphorylation of serine and threonine residues near the DNA binding site and phosphorylation of serines near the N-terminus.
Protein tyrosine phosphatases
A soluble protein phosphatase specific for phosphotyrosines (PTP1B) was first isolated from human placenta.2 Its amino acid sequence has stretches homologous with the tandem repeat domains present in the cytoplasmic portion of CD45 (leukocyte common antigen),3 a receptor-like protein
642
Protein Dephosphorylation and Protein Phosphorylation
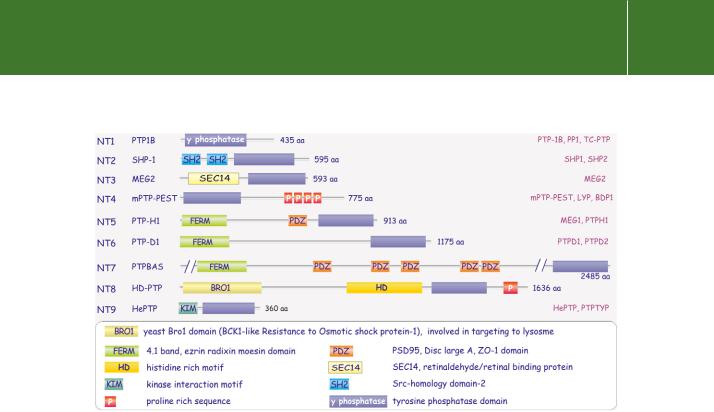
FIG 21.2 Domain architecture of cytosolic tyrosine phosphatase families. Nine distinct families of cytosolic tyrosine phosphatases are recognized on the basis of sequence differences in the phosphatase domain and of the presence of additional domains. Information from Andersen et al.4
expressed on cells of haematopoietic lineage. Using the DNA sequence that codes for the catalytic domain as a probe (the conserved ‘signature motif’), some 113 distinct vertebrate PTP catalytic domains have been identified, with37 distinct PTP genes in the human genome.
Sequence comparison of the catalytic domains of the vertebrate genes reveals |
For more information, |
17 subtypes that comprise both cytosolic (Figure 21.2) and transmembrane |
refer to the tyrosine |
phosphatases (Receptor-PTP) (Figure 21.3). The diversity of tyrosine protein |
phosphatase database |
phosphatases is greatly enlarged through alternative splicing, alternative |
http://ptp.cshl.edu or the |
promoter usage, and post-translational modification. Notably, as a result of |
parallel site http://science. |
alternative splicing, some of the receptor-PTP subtypes also contain cytosolic |
novonordisk.com/ptp). |
variants (for example PTP from the R4 group).4 |
|
In contrast to the serine/threonine and tyrosine kinases, which share a |
The many abbreviations |
used in this chapter are |
|
common ancestry, the tyrosine phosphatases and the serine/threonine |
collected together at the |
phosphatases bear no relation to each other. Unlike the serine/threonine |
end of the chapter. |
phosphatases, in which substrate specificity is determined by associated |
|
|
|
targeting subunits, the tyrosine phosphatases are all monomeric enzymes. |
|
The various domains that flank the catalytic domain act as targeting |
|
sequences and play important roles in determining the subcellular |
|
localization and the control of activity. How the structural diversity reflects |
|
differences in substrate recognition remains unclear. |
|
643
Signal Transduction
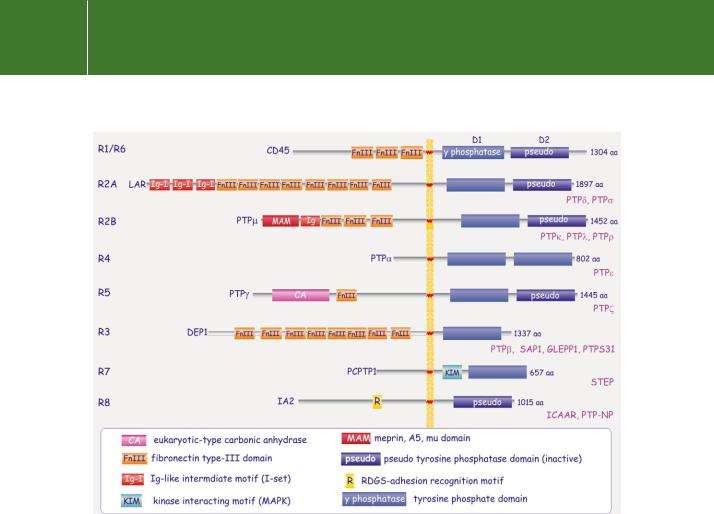
FIG 21.3 Domain architecture of receptor tyrosine phosphatases. All contain a tyrosine protein phosphatase motif, often two, D1 and D2, in tandem, and are distinguished by their extracellular domains. Some have elaborate extracellular structures resembling adhesion molecules or growth factor receptors, others appear rudimentary and ligand association is hard to imagine. No ligands have been identified for most of these ‘receptor-like’ phosphatases.
Cytosolic PTPs
The cytosolic PTPs are classified according to sequence homology of their catalytic domains. There are nine subclasses, each having different additional domains. An important subclass, comprised of SHP-1 and SHP-2, possesses SH2 domains, whereas others are characterized by the presence of prolinerich sequences in the vicinity of the C-terminus. Again, others have FERM and PDZ domains. We will return later to PTP1B, SHP-1, and SHP-2.
Included within the cytosolic PTP family are the dual-specificity phosphatases, which are active at both tyrosine and serine/threonine residues. Since the first of these to be identified was VH1, encoded by vaccinia virus, they are also referred to as VH1-like phosphatases.5 The dual-specificity phosphatases also have homology with Cdc25, a regulator of mitosis in fission yeast (Schizosaccharomyces pombe). This activates cyclin-dependent kinase-2
644

Protein Dephosphorylation and Protein Phosphorylation
by dephosphorylation of adjacent threonine and tyrosine residues.6 Dualspecificity phosphatases share the general signature motif but otherwise display little similarity with the phosphotyrosine-specific phosphatases (and are therefore not included in Figure 21.2). We return to dual specificity phosphatases on page 661.
Transmembrane receptor-like PTPs
Nearly all the transmembrane PTPs contain the tandem repeats D1 and D2, both of which express the catalytic signature motif. However, with the
exception of PTP , only the membrane-proximal D1 domains are catalytically active. The inactivity of the D2 domains can be ascribed to the absence of invariant amino acids (tyrosine and asparagine, see next section) that converge around the active site. The preservation of the D2 domain among species
and within nearly all receptor-like PTPs indicates that it probably serves an important physiological function. Its structural integrity is important for stability of the RPTP as a whole. Also, there is the possibility that D2 might be involved in the regulation of D1, or that it could assist in substrate recognition.
The transmembrane PTPs were originally classified on the basis of their extracellular ectodomains.7 Happily, when they are sorted according to the sequence homology of their catalytic domains, the categorization remains almost the same. (The initial classification has therefore been retained.) The ectodomains range from very short chains, having no apparent function, to extended structures with putative ligand-binding domains similar to those present in adhesion molecules (fibronectin repeats or immunoglobulin repeats, Figure 21.3). Such diversity suggests a wide range of biological functions which have, however, proved hard to pin down. This is largely because identification of their physiological substrates has been hampered by the non-specificity of these enzymes when assayed for activity in vitro. Moreover, although some are predicted to be receptors, their ligands have so far been elusive. PTP and PTP may take part in homotypic adhesion through their MAM domains.8,9 When expressed in insect cells, they induce Ca2 -independent cell aggregation, but even here the downstream pathways remain unclear. We will return later to CD45 and DEP1.
Tyrosine specificity and catalytic mechanism
The protein tyrosine phosphatases are without effect on phosphoserine or phosphothreonine residues, but there are a large number of phosphotyrosine proteins that act as substrates. This suggests that the overall structure of
the substrate is not the main determinant of selectivity; instead, substrate recognition is determined primarily by the presence of the pY residue in the context of its peptide environment. In particular, specificity results from the depth of the catalytic cleft (Figure 21.4b, c).
645
