
- •Protein tyrosine phosphatases
- •Cytosolic PTPs
- •Transmembrane receptor-like PTPs
- •Tyrosine specificity and catalytic mechanism
- •PTPs in signal transduction
- •PTP1B, diabetes, and obesity
- •PTP1B as a possible therapeutic target for the treatment of type 2 diabetes and obesity
- •Redox regulation of PTP1B: reactive oxygen species as second messengers
- •Regulation of SHP-1 and -2
- •SHP-1, JAKs, and STAT5
- •SHP-2 and the Ras–MAP kinase pathway
- •Insight through the Noonan syndrome
- •Density enhanced PTP (DEP1)
- •CD45 and the regulation of immune cell function
- •Regulating receptor PTPs
- •Dual specificity phosphatases
- •Regulation of MAP kinases by dual-specificity protein phosphatases (DS-MKP)
- •Physiological role of the dual-specificity MAP kinase phosphatases
- •Dual-specificity phosphatases in development
- •PTEN, a dual-specificity phosphatase for phosphatidyl inositol lipids
- •Serine/threonine phosphatases
- •Classification of the serine/threonine phosphatases
- •Regulation of PPPs
- •Phosphorylation of the catalytic subunits
- •Regulation by intramolecular domain interaction
- •Regulatory subunits of PP1
- •Inhibitors of PP1, PP2A, PP4, and PP5
- •PP1 in the regulation of glycogen metabolism
- •Regulation of glycogen metabolism: muscle
- •Regulation of glycogen metabolism: liver
- •PP2B (calcineurin)
- •Dephosphorylation of NFAT: immunophilins show the way
- •References
Protein Dephosphorylation and Protein Phosphorylation
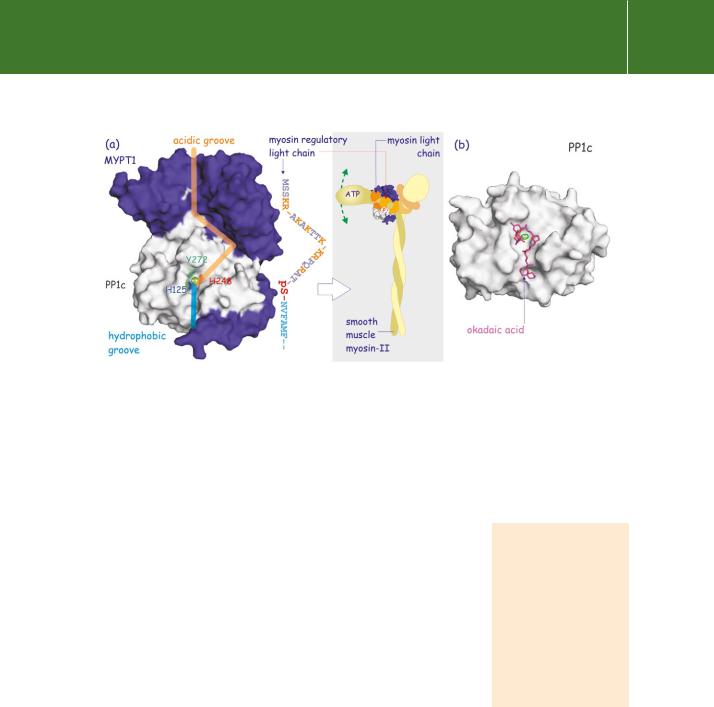
FIG 21.29 Regulation of PP1c by MYPT1 and inhibition by okadaic acid. (a) Attachment of the regulatory subunit MYPT1 to PP1c (the PP1 variant) creates an extended acidic groove on its surface which forms a perfect fit for the N-terminal sequence of the regulatory light chain of myosin II, which in turn has many basic residues (K or R in orange). At its other end, the groove is hydrophobic enabling it to accommodate a stretch of hydrophobic residues (blue) in the regulatory light chain. This causes it to align so that the phosphoserine lies above the catalytic pocket (indicated by ++). Thus MYPT1 increases the affinity of PP1δ some 10-fold for this particular substrate. Dephosphorylation of myosin regulatory light chain inactivates the ATPase activity of myosin-II and hence prevents movement of its head, resulting in muscle relaxation. (b) Okadaic acid, a causative toxin of diarrhetic shellfish poisoning in Europe, inhibits PP1c by occupying the catalytic cleft, preventing access of substrate. (1jk7,134 1s70133).
Inhibitors of PP1, PP2A, PP4, and PP5
PP1, PP2A, PP4, and PP5 are potently inhibited by the tumour promoters okadaic acid (figure 21.29b) and microcystin LR135. PP2B (calcineurin) is inhibited by the complex tacrolimus (FK506) FKBP12 (see page 683). Neither of these is capable of associating with the phosphatase alone, but together they form a stable system that inhibits its activity towards protein substrates while having no effect on the dephosphorylation of small model substrates (such as p-nitrophenol phosphate).136 In brain, PP2B forms
a complex with AKAP-79, an A-kinase anchoring protein that is a noncompetitive inhibitor of PP2B.137
PP1 in the regulation of glycogen metabolism
Site-specific phosphorylation of the glycogen targeting subunit (GM) of PP1c in muscle enables it to generate appropriate responses for adrenaline and Ca2 on the one hand (glycogenolysis), and for insulin on the other (glycogen synthesis).
Regulation of glycogen metabolism: muscle
In skeletal muscle, glycogenolysis is activated by adrenaline. It causes the production of cAMP leading to the activation of PKA (Figure 21.30a) and sets in
Myosin light chain kinase and myosin phosphatase regulate smooth
muscle contraction and relaxation. Phosphorylation of the myosin regulatory light
chain turns on the ATPase activity of myosin and this initiates the movement of actin filaments by the myosin motor proteins.
679
Signal Transduction
train a number of phosphorylation reactions. First it phosphorylates GM which destabilizes the complex by a factor of 104, allowing the dissociation of PP1c.138 PKA also phosphorylates the inhibitory subunit of PP1, I-1, and through this
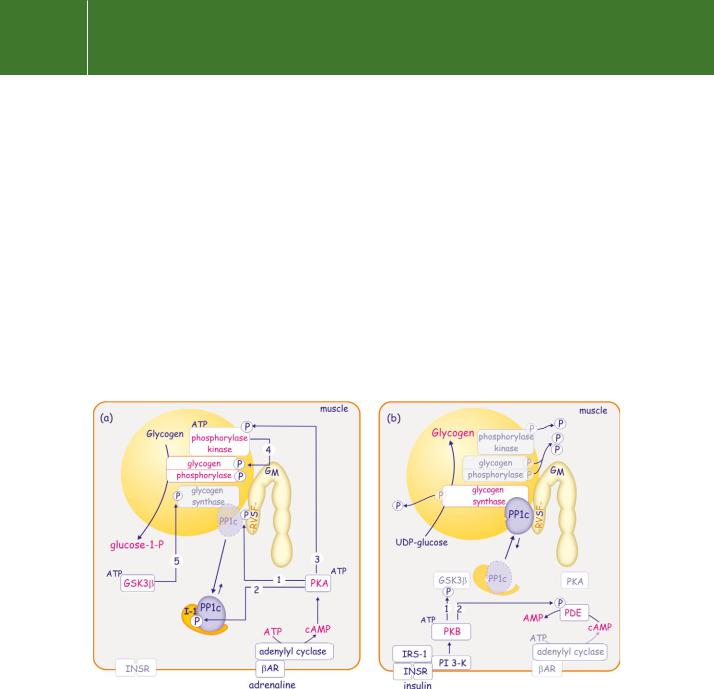
it creates a pseudosubstrate binding site. As a result, the liberated PP1c is hidden, unable to dephosphorylate proteins associated with glycogen. Finally, PKA phosphorylates and stimulates glycogen phosphorylase kinase. Because of the lack of PP1 activity, kinase activity now predominates. Phosphorylase kinase effectively phosphorylates and activates glycogen phosphorylase, while simultaneously GSK3 phosphorylates and inactivates glycogen synthase. The consequence of all this is glycogenolysis, the net breakdown of glycogen into numerous units of glucose-1-P.
Insulin brings about the reverse reaction (Figure 21.30b). It causes activation of PI 3-K, which in turn leads to activation of PKB. This inactivates GSK3 (see Figure 18.10, page 558). PKB also phosphorylates and activates cyclic
FIG 21.30 Decision-making in glycogen synthesis and breakdown: opposing effects of adrenaline and insulin. (a) Adrenaline drives glycogenolysis through the activation of PKA. This leads to phosphorylation of GM in the PP1c binding motif (-RVSF-, see Figure 21.25), causing them to dissociate (1). It also leads to phosphorylation of I-1 which, through the creation of a pseudo-substrate site, more effectively sequesters free PP1c (2). Finally PKA phosphorylates and activates glycogen phosphorylase kinase (3) which phosphorylates and activates glycogen phosphorylase (4). With the phosphorylation and inhibition of glycogen synthase by GSK3 (5), the balance shifts towards glycogenolysis with liberation of glucose-1-P. (b) Insulin stimulates glycogen synthesis. This is achieved by silencing the protein kinases above. PKB is activated and this phosphorylates and inactivates GSK3 (1). It also phosphorylates and activates phosphodiesterase (PDE) so depleting cAMP (2). With both GSK3 and PKA turned off, PP1c now dominates the scene. It inactivates both phosphorylase kinase and glycogen phosphorylase, and it activates glycogen synthase. The balance shifts towards glycogen synthesis (incorporation of UDP-glucose).
680

Protein Dephosphorylation and Protein Phosphorylation
nucleotide phosphodiesterase (PDE). As a result, the level of cAMP falls and PKA activity is suppressed. With the lack of both GSK3 and PKA activity, the phosphatase action of PPIc predominates, phosphorylase kinase and glycogen phosphorylase are inactivated, and glycogen synthase is activated. The balance shifts towards glycogen synthesis.
In order to switch from glycolysis to glycogen synthesis, PP1c is recruited to its regulatory GM subunit. This requires the action of PP2B or PP2A to dephosphorylate both GM and I-1 (Figure 21.31). This is another example of phosphatases acting as true reset buttons.139
Regulation of glycogen metabolism: liver
The mechanisms that control phosphatase (PP1) activity in liver and muscle are different. In liver, the activity of the GL subunit is not controlled by phosphorylation. Instead, inhibition of phosphatase activity, necessary to suppress glycogen synthase activity, is exerted by the phosphorylated (active) form of the target enzyme glycogen phosphorylase139 (Figure 21.32). This acts as an allosteric inhibitor of PP1c at extremely low concentrations. The interaction between the two is made possible because GL binds both enzymes, PP1c and
FIG 21.31 Rebalancing glycogen breakdown (PP2A and PP2B). When adrenaline is removed, glycogen metabolism is reset through dephosphorylation of the GM-regulatory subunit. This is mediated by PP2A and Ca2 -dependent PP2B (calcineurin). PP1c rejoins GM, and glycogen metabolism again becomes sensitive to adrenaline and insulin. Under these ‘resting’ conditions, it is likely that PP1c shuttles between GM and I-1, allowing a low basal release of glucose-1-phosphate.
681
