
- •Protein tyrosine phosphatases
- •Cytosolic PTPs
- •Transmembrane receptor-like PTPs
- •Tyrosine specificity and catalytic mechanism
- •PTPs in signal transduction
- •PTP1B, diabetes, and obesity
- •PTP1B as a possible therapeutic target for the treatment of type 2 diabetes and obesity
- •Redox regulation of PTP1B: reactive oxygen species as second messengers
- •Regulation of SHP-1 and -2
- •SHP-1, JAKs, and STAT5
- •SHP-2 and the Ras–MAP kinase pathway
- •Insight through the Noonan syndrome
- •Density enhanced PTP (DEP1)
- •CD45 and the regulation of immune cell function
- •Regulating receptor PTPs
- •Dual specificity phosphatases
- •Regulation of MAP kinases by dual-specificity protein phosphatases (DS-MKP)
- •Physiological role of the dual-specificity MAP kinase phosphatases
- •Dual-specificity phosphatases in development
- •PTEN, a dual-specificity phosphatase for phosphatidyl inositol lipids
- •Serine/threonine phosphatases
- •Classification of the serine/threonine phosphatases
- •Regulation of PPPs
- •Phosphorylation of the catalytic subunits
- •Regulation by intramolecular domain interaction
- •Regulatory subunits of PP1
- •Inhibitors of PP1, PP2A, PP4, and PP5
- •PP1 in the regulation of glycogen metabolism
- •Regulation of glycogen metabolism: muscle
- •Regulation of glycogen metabolism: liver
- •PP2B (calcineurin)
- •Dephosphorylation of NFAT: immunophilins show the way
- •References
Signal Transduction
Regulation of SHP-1 and -2
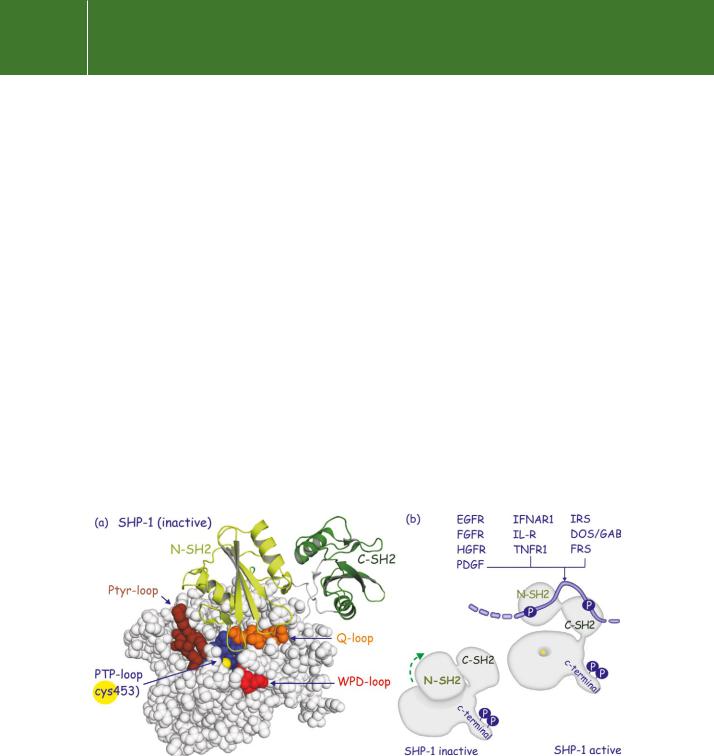
Structural analysis of SHP-1 has revealed that part of the N-terminal SH2 domain is wedged into the catalytic domain (Figure 21.10). This simultaneously obstructs the catalytic cleft and distorts the phosphotyrosine binding site. In this state activity is low. Binding of this SH2 domain to a pY-containing peptide can relieve some of the intramolecular inhibition, but full activity
is only achieved when both SH2 domains are engaged.53 This occurs when SHP-1 (or-2) binds to tandem phosphorylation sites on phosphorylated tyrosine kinase receptors, some of their substrates or tyrosine phosphorylated cytokine receptors54 (Figure 21.10).
SHP-1, JAKs, and STAT5
A role for SHP-1 was first indicated in mice having a somewhat motheaten appearance with patches of inflamed skin and loss of hair.56 This phenotype, arising from the absence of SHP-1, is due to a systemic autoimmune condition caused by abnormalities in cells of haematopoietic lineage and it is marked by a general overexpansion of cell numbers. The differentiation and functions of natural killer (NK) cells and erythroid cells are also adversely affected. There is an accumulation of macrophages and neutrophils in the lungs and the mice die within weeks of birth. Two naturally occurring point mutations are at the origin of the motheaten phenotype. The motheaten allele (me) generates an
FIG 21.10 Autoinhibition of SHP tyrosine phosphatases. (a) The most N-terminal SH2 domain of SHP-1 wedges into the catalytic cleft of the tyrosine phosphatase domain. One of the loops of N-SH2 contacts the PTP-loop region to obscure the pocket that leads to the catalytic Cys residue (yellow). (b) When the SH2 domains are bound to tyrosine phosphorylated proteins, the inhibitory restraint on SHP is relieved. Engagement of both SH2 domains induces maximal activity. Examples include receptors (EGFR, FGFR, HGFR, PDGFR, ILR, TNFR1) and the adaptors Dos/GAB and IRS (2b3o55).
654

Protein Dephosphorylation and Protein Phosphorylation
early frameshift and consequently homozygous me/me mice are protein-null (lethal after weeks). The viable motheaten allele (mev) encodes two aberrant SHP-1 proteins that possess 20% of the normal enzyme activity (lethal after months) (reviewed in Neel et al.52). On this basis, SHP-1 can be regarded as a negative regulator of signalling.
Biochemical and functional studies established this role in a wide range of signalling pathways downstream of receptors for ligands that include EPO, prolactin, CSF-1, EGF, BCR, and TCR (reviewed in Neel et al.52 and Zhang et al.57). There are multiple ways in which SHP-1 interferes, but the
common theme is that the phosphatase is invariably recruited into signalling complexes through its SH2 domains. This brings the SHP-1 into the proximity of the phosphotyrosyl groups of receptors, recruited cytoplasmic kinases,
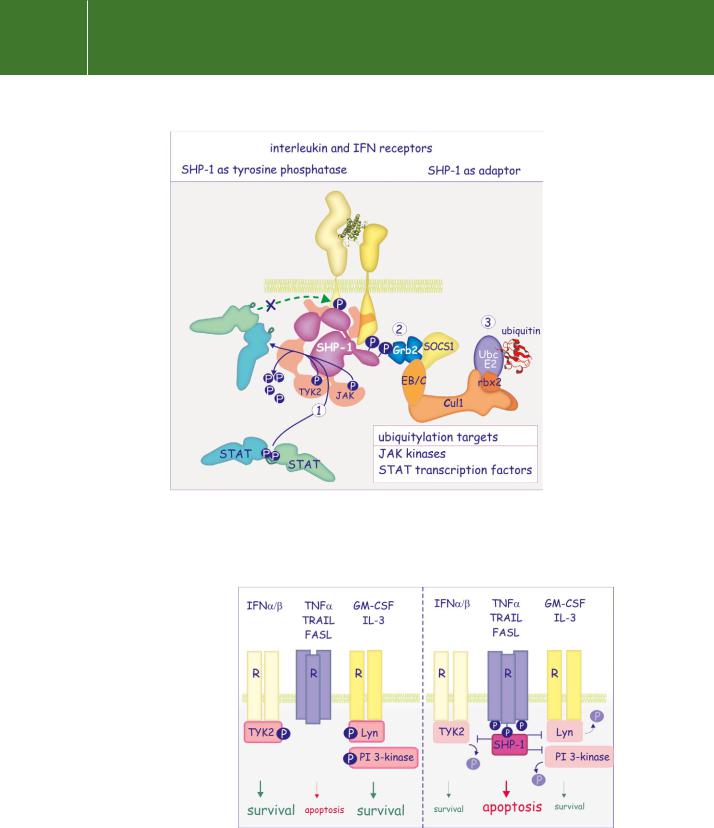
or multiphosphorylated docking proteins. Moreover, SHP-1 itself can be phosphorylated on its C-terminal tyrosine residues, which converts it into an adaptor. Figure 21.11 illustrates the diverse actions of SHP-1.
SHP-1 also enhances the sensitivity of cells to apoptosis (Figure 21.12). Neutrophils from mice possessing just one me allele (me / ) are somewhat resistant to apoptosis and this might contribute to the increased number of white blood cells in the motheaten phenotype. SHP-1 binds to the receptors Fas and TNF- , both of which contain a conserved cytoplasmic phosphorylation motif that is recognized by SHP-1, causing its activation. Activated SHP-1 then sensitizes the cell for apoptosis, possibly by counteracting the protective activity of the kinase Lyn or TYK2.58
Lastly, 1- and 2-integrin-mediated adherence to the extracellular matrix is enhanced in bone-marrow macrophages deficient in SHP-1. These cells also show enhanced uptake of opsonized red blood cells, which in part involves the 2-integrins (Mac-1), and it is therefore suggested that SHP-1 negatively regulates ‘inside-out’ and ‘outside-in’ integrin signalling. Again, the targets of SHP-1 remain unclear.
SHP-2 and the Ras–MAP kinase pathway
Targeted disruption of the gene encoding SHP-2 in mice causes early embryonic death, but other than concluding that it is important, little can be learned from this. Early indications about a possible role of SHP-2 in cell signalling came from three lines of evidence.
First, the gene is homologous to Drosophila Corkscrew (Csw). The Corkscrew pathway directs the differentiation of the terminal, non-segmented regions of the fly embryo.59 Csw is involved in activation of the serine/threonine kinase Draf (equivalent to mammalian Raf). Draf is activated through the Dras pathway and this implies that the phosphatase provides a positive signal downstream of receptor tyrosine kinase activation. Molecular and genetic analyses indicate that the Dos protein is a likely substrate.60 This contains a
655
Signal Transduction
FIG 21.11 SHP-1 mediated inhibition of receptor functions. Direct recruitment of SHP-1 to cytokine or growth factor receptors causes dephosphorylation in the activation segment and inactivation of JAK. This is followed by dephosphorylation of STAT transcription factors and of the receptor (1). Tyrosine phosphorylated SHP-1 can also act as an adaptor to recruit Grb2/SOCS1 into the receptor signalling complex (2). SOCS1, in turn, recruits an E3-ubiquitin ligase complex to the cytokine receptor (3) and sets in train the ubiquitylation and subsequent destruction of JAK and STAT.
FIG 21.12 SHP-1 in sensitization of neutrophils to apoptosis. SHP-1 binds to tyrosine phosphorylated Fas or TNFreceptors and then interferes with Lynand Tyk2-mediated tyrosine phosphorylation of downstream effectors. In this way it blocks survival signals and renders neutrophils more sensitive to apoptosis.
656
Protein Dephosphorylation and Protein Phosphorylation
PH domain which permits membrane association, and numerous tyrosine phosphorylation sites through which it could act as a membrane-bound docking protein involved in relaying signals from Sevenless (an insulin-like rPTK) to Ras1 (see page 327).
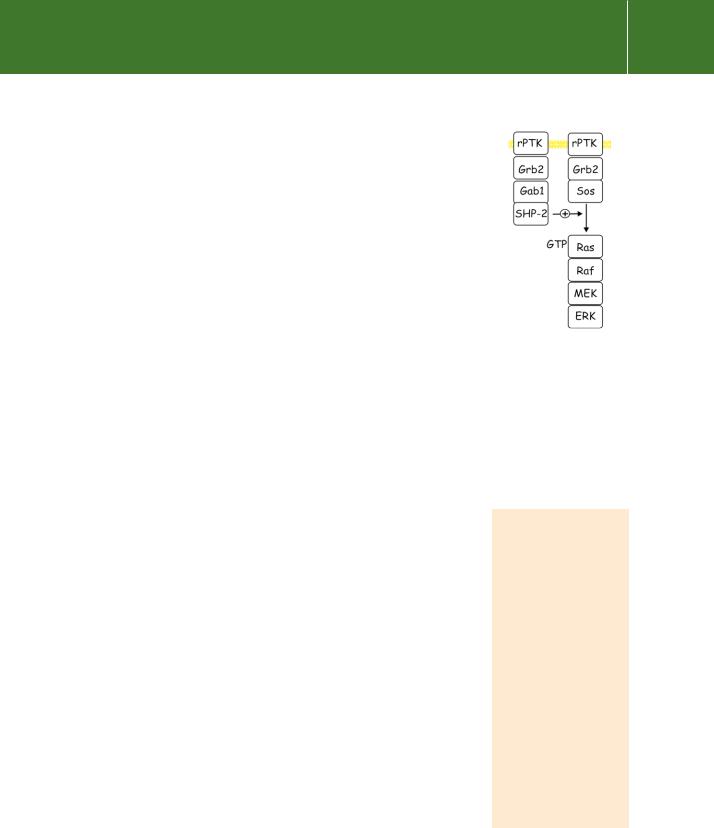
Gab1, a human protein that resembles Dos, is also associated with SHP-2. In SHP-2 / embryonic stem cells there is an enhancing effect of SHP-2 on EGF signalling which requires the concerted action of Gab1 and an unidentified tyrosine phosphorylated protein p90 (Figure 21.13).61
Secondly, expression of a mutant form of SHP-2 lacking the catalytic domain in Xenopus embryos causes tail truncations and prevents animal cap elongation (short toad, composed mainly of head structures).62 These processes are determined in part by the receptor for FGF which also signals through the MAP kinase pathway and so the phosphatase SHP-2 must act between the FGF-R and the activation of MAP kinase. Similarly, fibroblasts expressing the catalytically inactive form of SHP-2 fail to activate MAP kinase in response to FGF, PDGF or insulin-like growth factor (IGF).63
Thirdly, in mice there is an essential role for SHP-2 in limb development and in formation of the branchial (pharyngeal) arch, two other pathways controlled by FGFR signalling.64
Insight through the Noonan syndrome
Missense mutations in the SHP-2 gene have been identified as the underlying cause of Noonan syndrome. Most of the altered amino acid residues are located in or around the interacting surfaces of the N-terminal SH2 domain and the catalytic domain, and are predicted to relieve the intramolecular inhibition caused by binding of the SH2 domain to the catalytic domain.65
In some cases there are mutations in residues present in the C-terminal SH2 domain and in the peptide linking the two SH2 domains. However, there is more to the Noonan story because there is no absolute correlation between basal activities of the SHP-2-mutants and the disease they induce. Some mutations, even those associated with severe symptoms, are without effect on the basal activity or on phosphotyrosine-mediated activation.66
Gain-of-function mutations in SHP-2 are linked to some leukaemias.70 This is perhaps not surprising, given its stimulatory role in the Ras–MAP kinase pathway. Despite some uncertainty with respect to the exact mechanism of cell transformation, SHP-2 mutants provided the first example of a gain-of- function mutation in a PTP that is the underlying cause of a human disease.
Density enhanced PTP (DEP1)
For DEP1, matters are just a bit clearer. This phosphatase was originally discovered by probing a HeLa cell cDNA library with a PCR fragment encoding the catalytic domain of a putative PTP. The receptor-type PTP isolated is
FIG 21.13 SHP-2, its role in activation of the Ras–MAP kinase pathway. Genetic and biochemical analyses place SHP-2 between Sos and Ras. How it stimulates GTP loading of
Ras and so the MAP kinase pathway remains unknown.
Noonan syndrome is an autosomal dominant disorder characterized by short stature and multiple developmental abnormalities including congenital heart and skeletal malformations.67,68
The incidence of this syndrome is high – 1 in 1000–2500 live births
– and in a survey of more than 100 unrelated cases about 50% displayed
a mutation in SHP-2. There is an even higher incidence of mutations among familial cases.69 All these are missense mutations.
657
