
- •Accessory and pseudo receptors: betaglycan, endoglin, cripto, and BAMBI
- •Betaglycan
- •Cripto
- •BAMBI
- •Downstream signalling: Drosophila, Caenorhabitidis, and Smad
- •Smad proteins have multiple roles in signal transduction
- •Receptor-regulated Smads 1, 2, 3, 5 and 8: receptor recognition
- •Cytoplasmic retention of receptor-regulated Smad proteins
- •Common mediator Smad4
- •Hetero-oligomeric complex formation
- •Smad–Smad complexes
- •Nuclear import and export
- •SMAD transcriptional complexes
- •Activation of gene expression
- •Repression of gene expression
- •A self-enabling response: repression of myc is prerequisite for expression of cell cycle inhibitors
- •The Smad linker region: hotspot for kinases and an E3-ligase
- •Smurf-mediated Smad degradation
- •Inhibitory Smads
- •BAMBI, a signal inhibitory pseudo receptor
- •Smad phosphatases
- •References

Chapter 20
Signalling Through Receptor
Serine/Threonine Kinases
The TGF family of growth factors
The receptor serine/threonine protein kinases1 are all dedicated to relaying signals deriving from members of the transforming growth factor family
(TGF ). The first of these to be identified, TGF 1, emerged as a transforming factor for mesenchymal cells (see page 304). Related proteins were revealed through loss-of-function mutation studies in Drosophila (Dpp gene) and
Xenopus (Vg1 gene).
We now know that TGF is a member of a family of structurally related proteins, most of which have little or nothing to do with cell transformation. With 42 members in the human genome, 7 in Drosophila, and 4 in Caenorhabditis elegans, the TGF family is one of the most prominent families of first messengers.2 The mammalian TGF family can be divided into a number
of subfamilies: TGF itself, BMP, GDF, activin, inhibin, nodal, myostatin, and AMH. These factors may be produced by many cell types (TGF ), or just
a few (myostatin). They may be active from the earliest stages of embryo development through adulthood (BMP), or for only very limited periods (AMH).3
The many abbreviations used in this chapter are collected together at the end of the chapter.
599

Signal Transduction
Myostatin and big burgers
Myostatin, a member of the TGF family, is a negative regulator of muscle growth. It was first identified in nullmutant knockout mice
that exhibit a widespread increase in muscle mass due to hyperplasia (fibre number) and hypertrophy (fibre thickness). An 11 bp deletion in the C-terminal coding region of the myostatin gene (Mstn) in the Belgian
Blue cattle breed and a missense mutation in the Piedmontese
breed are responsible for their double-muscled phenotypes4 (Figure 20.1). The animals are apparently born with
a normal phenotype, the so-called double muscling only appearing at about 4–6 weeks. It is not uncommon for the bulls to achieve a weight exceeding 1300 kg. The animals are docile and the beef is said to be delicious. An inherited
mutation in the myostatin gene, boosting muscle growth and reducing fat, also occurs in humans. It may come as no surprise that products that claim to regulate myostatin
are already used by many athletes and bodybuilders.
Fig 20.1 Big beef.
Springhill Wizzard, owned by Martin Brothers, Newtownards, was the best Belgian Blue Junior Bull and Reserve Male Champion at the Balmoral Show 2007. Thomas and James Martin are pictured exhibiting the prizewinner. Photograph by kind courtesy of Columba O’Hara and the British Blue Cattle Society.
Several members of the TGF family are released from cells as dimers still attached (non-covalently) to their propeptides (Figure 20.2a). These ‘latent’ complexes are often linked to one of the several TGF -associated proteins, also termed latent.5 Release of the ligand occurs through proteolytic activity (by plasmin or metalloprotease MMP-2 or -9) or by interaction with thrombospondin or the integrins V 6 and V 8 (which modify the propeptide by binding to an RGD sequence). The importance of integrinmediated activation of TGF is illustrated by the finding that lack of the
RGD motif, and thus failure of the interaction with integrins, results in organ wasting similar to that observed in mice that do not express TGF 1.6,7 The general idea is the existence of a matrix-associated ligand-reservoir, ready to be activated under favourable conditions.
Sequestration of TGF family members also occurs through ligand traps such as chordin, DAN/Cerberus, decorin, follistatin, noggin, or 2M (see Figure 20.9). We will return to chordin and noggin, which, by preventing BMP signalling, play an important role in the formation of neuroectoderm in Xenopus laevis.
TGF receptors, type I and type II
TGF 1 came to notice in a search for transforming growth factors and initially, it was anticipated that its receptor would be linked, either directly
600
Signalling Through Receptor Serine/Threonine Kinases
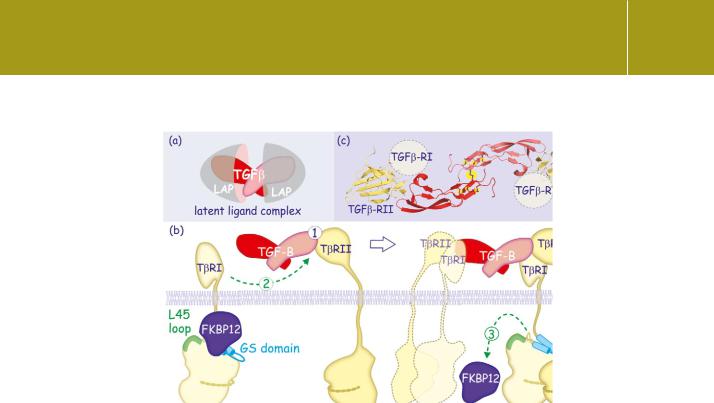
Fig 20.2 TGF induces receptor dimerization.
(a) The TGF 1-ligand constitutes a dimer associated with its propeptides (LAP). To bind its receptor, the ligand detaches from LAP. Different modes of activation include degradation by proteases such plasmin, MMP-2 or -9 but also through contact with thrombospondin or the integrins v 8 or v 8 (vitronectin receptor). (b) TGF 1 binds to the type II receptor (1) and this allows it to be recognized by the type I receptor (2). The inhibitory immunophilin FKBP12 detaches (3) and the type I receptor is phosphorylated by the type II (4). The activated type I receptor signals onward into the cell. Because TGF 1 is itself a dimer, two receptor complexes can form around the growth factor. (c) Molecular structure of TGF 3 associated with two type II receptors.
The presumed orientation of the type I receptor is indicated. The cysteine knot composed of numerous intramolecular disulfide bonds is indicated in yellow (1 ktz13).
or indirectly, to tyrosine phosphorylations. When it was later found that it inhibits proliferation of epithelial cells, interest shifted to the possibility that it prevents growth factor signalling. Neither of these ideas proved very fruitful. TGF 1 has no effect on tyrosine phosphorylation, nor (at least when measured on a time scale of minutes) does it affect the early events in EGF or PDGF receptor signalling. So it remained until 1990, when a receptor for activin was cloned and found to contain a putative transmembrane serine/threonine protein kinase domain.8 Similar domains were then found in two of the TGF 1 receptors.9–11
Based on their structural and functional properties, the receptors for this family of ligands are divided into subfamilies types I and II (T R-I and T R-II) (Figures 20.2b and 20.3).12 They are very similar. Both receptors are glycoproteins. Both have a single membrane span and an intrinsic serine/threonine kinase domain in the intracellular C-terminal segment. What distinguishes the type I receptor is a highly conserved stretch of 30 residues, the GS domain,
601

Signal Transduction
Fig 20.3 Domain architecture of TGF , its receptors and associated proteins.
The types I and II receptors contain a serine/threonine protein kinase domain and a cysteine-rich TGF - binding domain. The type I receptor
contains an additional GS domain and a number of phosphorylation sites that are involved in its activation by the type II receptor (which is constitutively active). Other abbreviations may be found in the list at the end of the chapter.
immediately preceding the kinase domain that regulates T R-I kinase activity. This stretch is attached to the kinase domain but is rather flexible and it can fluctuate between attached and detached states. This would allow the receptor to switch between inactive and active conformations. This
is prevented by the inhibitory immunophilin FKBP12 which holds the GS domain firmly in place. (Figures 20.2b and 20.3). Phosphorylation of the GS domain by the type II receptor breaks the inhibitory spell.
602

Signalling Through Receptor Serine/Threonine Kinases
As it has no GS domain, the type II receptor escapes inhibitory control and is therefore constitutively active, always in wait for the approach of the type I receptor.
The mammalian receptors are homologous with the punt (put), thickveins (tkv), and saxophone (sax) genes of Drosophila and with the dauer phenotypes 1 and 4 (daf-1 and daf-4) genes of C. elegans. The downstream signalling pathway from the TGF 1-receptors was revealed by searching for mammalian homologues of their counterparts in these organisms. It became apparent that mammalian TGF 1 receptors transmit signals into cells through the Smad proteins, a unique set of transcription factors (see below).14
Here we outline some of the mechanisms by which members of the TGF family of receptors elicit their effects on target cells. We concentrate on the pathway activated by TGF 1 through the T R-I and T R-II receptors and in particular on the pivotal role of the Smads in relaying signals to the nucleus. It will become apparent that these pathways are rather similar to those already described for the activation of the STATs through the tyrosine kinase-containing receptors such as those for EGF, PDGF, and interferon (see page 353). The main theme is that a receptor complex first recruits and then phosphorylates a transcription factor. This forms an oligomeric complex that translocates to the nucleus to interact with DNA-response elements in promoter regions of genes.
TGF -mediated receptor activation
TGF 1 is a disulfide-linked homodimer. It gathers pairs of type I and II receptors to form heterotetrameric receptor complexes (Figure 20.2). Ligandindependent homo-oligomers may exist, but do not transmit signals. TGF 1 can bind to T R-II in the absence of T R-I but not vice versa. TGF 1, however, cannot signal into the cell in the absence of T R-I. All this indicates that the most likely sequence of events is that TGF 1 first binds to T R-II, altering
its conformation so that it can then be recognized by T R-I. (In contrast, BMPs -2 and -4 first bind the type I receptors and then the type II). When the ligand brings the two receptors in close proximity, the type II receptor
phosphorylates the serines and threonines in the sequence TTSGSGSGL of the GS domain of T R-I (see Figures 20.2b and 20.5).
As with the tyrosine kinase-containing receptors, oligomerization and phosphorylation together constitute the signal for recruitment of effector proteins. However, the receptor serine/threonine kinases employ a quite different approach in the control of kinase activity. Here phosphorylation alters catalytic competence by removing an inhibitory constraint (the GS domain that wedges into the N-lobe) as well as by preventing binding of the inhibitor FKBP12 (Figure 20.4).15 Moreover, the detached phosphorylated GS domain now acts as a docking site for Smad proteins, the substrates of the type I receptors (Figure 20.5). This general mechanism applies to all TGF and BMP receptors, from
Drosophila to mammals.
603

Signal Transduction
Fig 20.4 Activation of the kinase domain of the TGF receptor. The structure of the TGFβ type I
receptor reveals a kinase domain. The activation segment (red) is structured but the presence of the GS domain (light blue), wedged into the N-lobe, causes a rotation of the αC-helix and thus a separation between K232
and E245. This prevents the correct positioning of ATP in the cleft. The threonine and three serine residues phosphorylated in the GS domain are shown as sticks in an orange ellipse. In order to achieve catalytic competence the GS domain detaches partially
(1), so allowing K232 and E245 to approach each other (2), allowing ATP to locate correctly. The L45 loop, which interacts with the L3 loop of Smad proteins is purple.
Fig 20.5 Schematic view of TGF receptor activation.
The TGF type I receptor exists in three different states. Inactive has GS wedged into the N-lobe (held in place by FKBP12); intermediate allows oscillation between the attached and partly-detached positions of GS; in the active state the phosphorylated GS cannot wedge into the N-lobe. Phosphorylation
is induced by the close proximity of the types I and II receptors. The phosphorylated GS, together with the L45 loop, form the binding site of receptorregulated Smad proteins (R-Smad) (1), which are subsequently phosphorylated on two serine residues at their C-termini (2). SARA facilitates the interaction between R-Smads and the type I receptor. Once phosphorylated, R-Smad proteins detach from the receptor (3). FYVE, MH1 and MH2 indicate protein domains: see text.
604

Signalling Through Receptor Serine/Threonine Kinases
Accessory and pseudo receptors: betaglycan, endoglin, cripto, and BAMBI
The quest for cell surface TGF -binding proteins revealed a third set of receptors, quite distinct from the T Rs I and II. Their intracellular domains are devoid of any sequence motif that could be involved in signal transduction. Betaglycan, endoglin, and cripto are coreceptors that support signalling. BAMBI is a pseudo-receptor that inhibits signalling.
Betaglycan
Betaglycan is a transmembrane proteoglycan that binds isoforms of TGF .16 This is vital in the case of TGF 2, as it facilitates its binding to the receptor, leading to the formation of a ternary complex comprising TGF 2, T RII, and betaglycan.17 T RI then binds to the complex, displacing the betaglycan. Expression of betaglycan in cells normally lacking this coreceptor causes an increase in TGF 2 binding and a concomitant increase in sensitivity to the ligand (Figure 20.6a).18
Inhibins and activins were first identified as factors that respectively suppress or stimulate secretion of FSH from pituitary gonadotropes.19 This functional antagonism is explained by the finding that they both bind the activin receptor ActR-II. Binding of activin promotes the recruitment of the activin type I receptor, ActR-IB, to initiate the signal. Inhibin recruits betaglycan
to form a stable complex so that there is a loss of the activin signal due to depletion of available ActR-II. This puts a block on further proceedings20 (Figure 20.6b). Expression of betaglycan in cells that normally respond poorly enhances the sensitivity to inhibin.
Endoglin
Endoglin, originally discovered as a glycoprotein antigen highly expressed on human endothelial cells,23 facilitates the interaction of TGF 1 already bound to T RII with ALK-1 (type -I receptor) in endothelial cells24 (Figure 20.6c). Thus
it determines the balance between ALK-1 signals, leading to proliferation and migration of endothelial cells, and TβRI (also referred to as ALK-5) signals that inhibit proliferation and migration. Since both receptors are expressed on endothelial cells, the presence of endoglin decides the outcome of the response to TGF , favouring an ALK-1-mediated proliferative and invasive response. It facilitates the formation of new blood vessels (angiogenesis).25,26 Inherited absence of functional endoglin is associated with the disorder haemorrhagic telangiectasia type I (HHT1), characterized by a leaky and poorly developed vascular bed.27
Cripto
Cripto is a small extrinsic membrane protein of the EGF–CFC gene family, members of which take part in embryonic anterior-posterior axis
In vivo, the functional role of betaglycan as a coreceptor is vital. Mice lacking betaglycan die during embryogenesis with
heart and liver defects,21 and targeting betaglycan during development disrupts mesenchyme formation in the
heart and branching morphogenesis in the lung.22 In the adult, loss of betaglycan is associated with tumour formation.
The receptor T RI was first identified biochemically, as a
component necessary for the transmission of TGF signals, but molecular characterization was spearheaded by the activin receptors. Sequences with high similarity were named activin-like kinases or ALKs. This explains the double nomenclature of T Rs and ALKs.
605
Signal Transduction
development.28 It binds to nodal, promoting the formation of active receptor dimers (ActRIB with ActRIIB) and also binds to activin, when attached to ActRII, but here it impedes access to the type I receptor (Figure 20.6d). Its tumour promoting action may in part be explained by its capacity to block activinmediated growth inhibition of epithelial cells.29
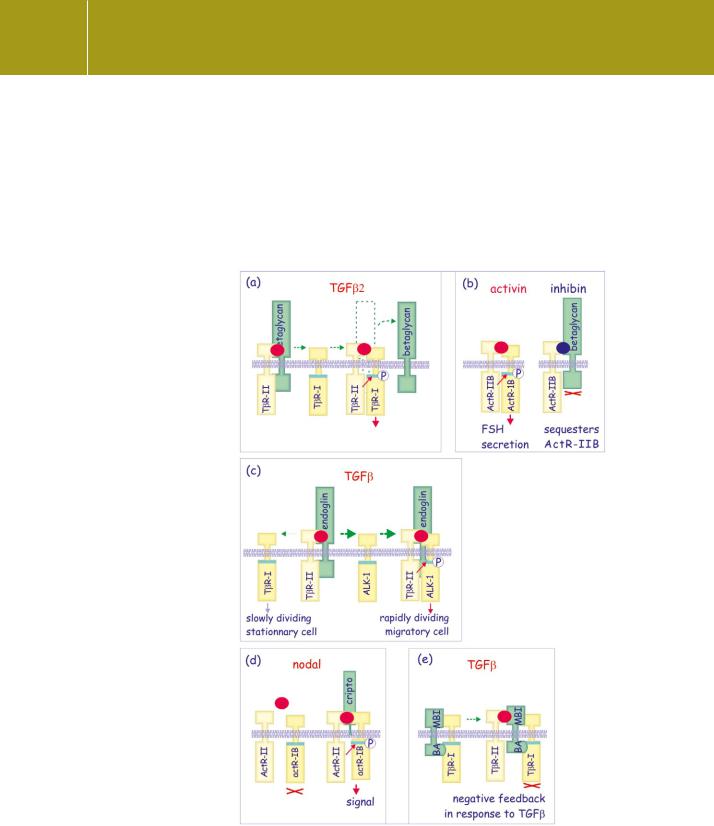
Fig 20.6 Accessory and pseudoreceptors.
Several membrane proteins bind the TGF family of ligands. None of these has intracellular signalling motifs; instead, they reinforce or inhibit signalling by affecting the interaction between the ligand and its receptors.
(a) Betaglycan binds TGF and facilitates its access to T R-II. Once a complex is formed with the type I receptor (TbR-I), it detaches.
Betaglycan is indispensable for TGF 2- mediated signalling. (b) Inhibin
binds betaglycan and ActR-IIB, and prevents the interaction with ActR-1B (Alk-4) resulting in formation of non-functional type II receptors. (c) Endoglin facilitates the formation
of T R-II/ALK-1 complexes and in endothelial cells it shifts the balance towards an ALK-1 response (rather than an TbR-I response). (d) Cripto is bound to the membrane by a glycosylphosphatidylinositol anchor.
It binds activin attached to ActRII and blocks signal propagation.
(e) Expression of BAMBI is induced by TGF signalling. It forms a complex with T R-I and prevents its phosphorylation by T R-II, thereby
blocking the TGF response. Red spots represent TGF 2, activin, or TGF in general; the dark blue spot is inhibin.
606
