
- •Protein tyrosine phosphatases
- •Cytosolic PTPs
- •Transmembrane receptor-like PTPs
- •Tyrosine specificity and catalytic mechanism
- •PTPs in signal transduction
- •PTP1B, diabetes, and obesity
- •PTP1B as a possible therapeutic target for the treatment of type 2 diabetes and obesity
- •Redox regulation of PTP1B: reactive oxygen species as second messengers
- •Regulation of SHP-1 and -2
- •SHP-1, JAKs, and STAT5
- •SHP-2 and the Ras–MAP kinase pathway
- •Insight through the Noonan syndrome
- •Density enhanced PTP (DEP1)
- •CD45 and the regulation of immune cell function
- •Regulating receptor PTPs
- •Dual specificity phosphatases
- •Regulation of MAP kinases by dual-specificity protein phosphatases (DS-MKP)
- •Physiological role of the dual-specificity MAP kinase phosphatases
- •Dual-specificity phosphatases in development
- •PTEN, a dual-specificity phosphatase for phosphatidyl inositol lipids
- •Serine/threonine phosphatases
- •Classification of the serine/threonine phosphatases
- •Regulation of PPPs
- •Phosphorylation of the catalytic subunits
- •Regulation by intramolecular domain interaction
- •Regulatory subunits of PP1
- •Inhibitors of PP1, PP2A, PP4, and PP5
- •PP1 in the regulation of glycogen metabolism
- •Regulation of glycogen metabolism: muscle
- •Regulation of glycogen metabolism: liver
- •PP2B (calcineurin)
- •Dephosphorylation of NFAT: immunophilins show the way
- •References
Signal Transduction
The leptin receptor is a cytokine receptor. It resembles the
granulocyte–monocyte colony stimulating factor (GM-CSF) receptor and signals mainly through the JAK2/STAT3 pathway. It also activates the IRS2–PI 3-kinase and Ras–MAPK pathways.
Insulin and leptin act on neurons in the hypothalamic arcuate
nucleus to regulate the desire to eat. The two hormones signify positive energy balance and satiety, manifested by (among other changes) increased release of pro-opiomelanocortin (POMC) peptides
and reduced release of NPY and AgRP. The consequence is
suppression of appetite and a greater propensity to undertake physical activity. In contrast, depletion of fat stores gives rise to low basal insulin and low leptin levels, which favour food intake and economy
of energy expenditure. Disruption of insulin and/or leptin signalling encourages food intake, reduces energy
expenditure, and leads to an increase in body mass. Stimulating insulin and/or leptin signalling does the reverse.
pathway.25,26 This is a good example of how feedback mechanisms prevent excess signalling (often referred to as the robustness of the system).
Introduction of antisense oligonucleotides to PTP1B into mice has the effect of suppressing PTP1B gene expression in the liver and adipose tissue, but not in muscle. Obese (ob/ob, leptin-deficient) and late onset diabetic (db/db, leptin receptor deficient) mice, treated in this way, exhibit enhanced sensitivity to insulin but maintain normal levels of blood glucose.27 This, and the resistance to obesity, has made PTP1B an attractive target for treating type 2 diabetes as well as obesity in general. The quest for inhibitors of PTP1B has attracted much attention and more than 40 crystal structures of complexes of PTP1B with inhibitors or substrates have been deposited in the Protein Data Bank.
PTP1B as a possible therapeutic target for the treatment of type 2 diabetes and obesity
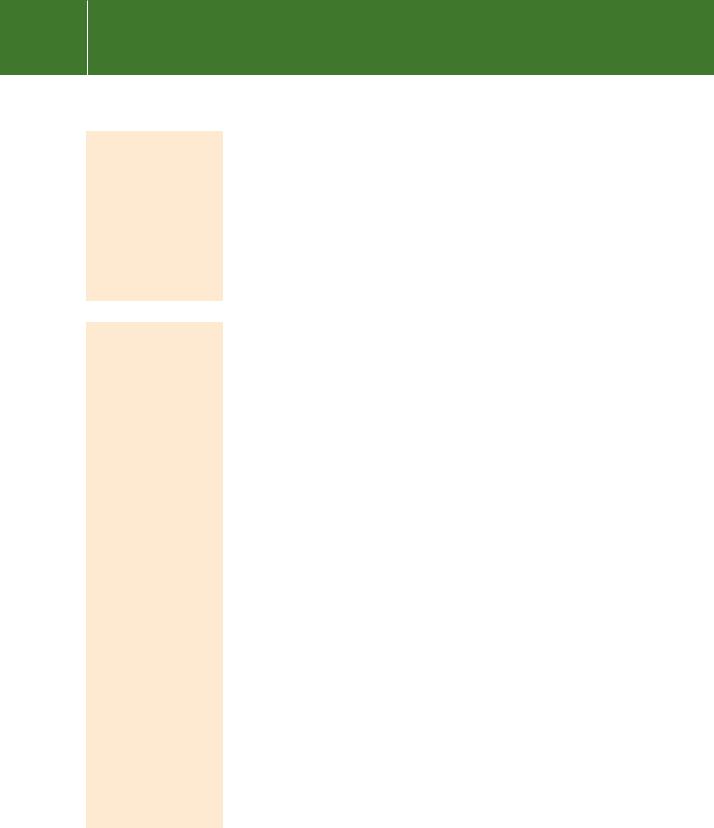
Optimal substrate recognition by PTP1B requires the amino acid motif D/E-pY- pY-R/K, also present in the kinases Trk, FGF-R, NGF-R, and Axl, and of particular relevance here, members of the JAK family.28 With respect to obesity, the JAKs are instrumental in signalling by the satiety hormone leptin via its receptor OB-RL (for a review, see Tartaglia29). JAK2, a physiological substrate of PTP1B, phosphorylates two residues on human OB-RL and probably acts to regulate leptin signalling (blocking the satiety signal). Hypothalamic neurons derived from PTP1B / knockout mice display markedly increased leptin-induced STAT3 phosphorylation (STAT3 is the transcription factor involved in the leptin response) (Figure 21.7). Since leptin-activated JAK2 is a substrate of PTP1B but not STAT3
or the leptin receptor itself,30 it follows that PTP1B negatively regulates leptin signalling. This provides a mechanism by which it may regulate obesity.31,32
Redox regulation of PTP1B: reactive oxygen species as second messengers
Numerous cells, including phagocytes, epidermal cells, fibroblasts, vascular smooth muscle cells, and osteoblasts, generate reactive oxygen species (ROS) in response to environmental cues.
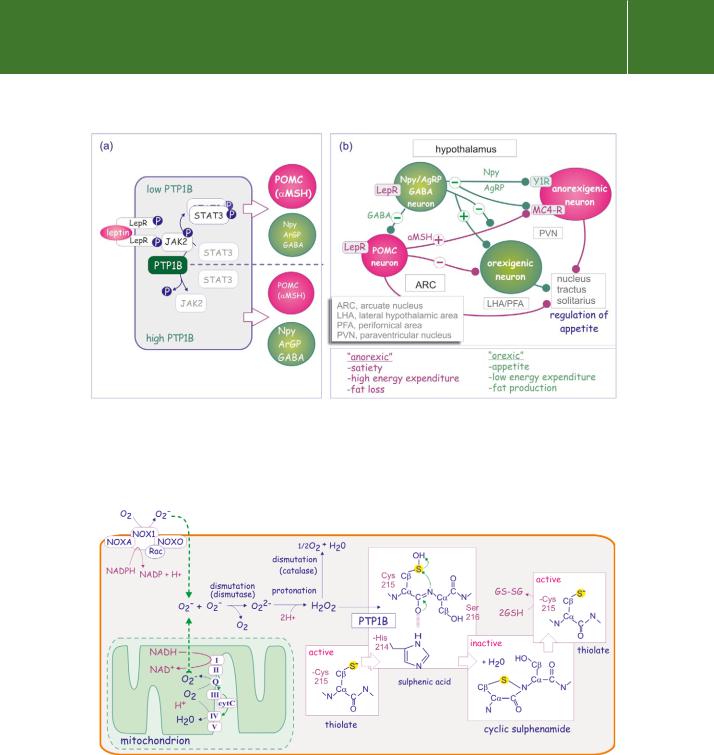
ROS originate from two different sources. The first is production by the NADPH-oxidase complex (NOX). In response to the GTPase Rac1, the subunit NOX1 transfers electrons across the plasma membrane delivering them to the acceptor O2, generating superoxide (O2 ) (Figure 21.8). TGF 1, IL-1, TNF- , insulin, PDGF, EGF, angiotensin, thrombin, and lysophosphatidic acid are amongst the cytokines that activate the NOX pathway33–35 (for a review see Chiarugi and Cirri36).
A second source of ROS, operational in all cell types, is the mitochondrial electron transport chain. Here, superoxide is released at the level of
650
Protein Dephosphorylation and Protein Phosphorylation
FIG 21.7 Role of PTP1B in satiety signalling. (a) High fat (or low fat turnover) releases leptin, a satiety signal, from adipose tissue. This binds to its receptor, LepRR, in neurons of the arcuate nucleus of the hypothalamus, and activates JAK2. This phosphorylates STAT3, which dimerizes and enters the nucleus, there to alter gene expression leading to an enhanced expression of POMC products ( MSH, CART, and CRH), and reduced expression of Npy, AgRP and GABA. (b) This translates into a predominantly anorexigenic signal (red neurons), leading to a feeling of satiety, high energy expenditure and loss of fat. Deletion of PTP1B renders mice resistant to obesity.
FIG 21.8 Generation of superoxide (O2 ) by NADPH oxidase and the mitochondrial electron transport chain. Superoxide (O2 ) is produced by NADPH oxidase, a complex comprising NOXA (NOX activator), NOXO (NOX organizer), Rac, and NOX1. It is also released from mitochondria. It is converted to oxygen and peroxide (O22 ), to become H2O2, which then transiently converts the active site cysteine thiolate into a catalytically inactive cyclic sulfenamide. Glutathione is required to restore enzyme activity. This reaction scheme applies for cysteines with a low pKa.
651

Signal Transduction
FIG 21.9 Stimulation of production of ROS by growth factors, hypoxia, oncogenes, and integrin binding. Various growth factors, cytokines, oncogenes, and integrins, as well as hypoxia, enhance production of ROS. Hydrogen peroxide causes inactivation of the active site cysteine of PTP1B. The transient inhibition enhances and prolongs phosphorylation of receptor tyrosine kinases and amplifies the growth factor signal. Other targets of H2O2 are proteins having an acidic cysteine residue (pKa5.4) such as other tyrosine phosphatases (PTP) and the transcription factors NF- B, AP1, and HIF.
ubiquinone (which normally shuttles electrons from complex II to III) (Figure 21.8). This plays a role in apoptosis, but transient production also occurs in response to other cues under conditions where apoptosis does not occur. Examples are hypoxia, the expression of oncogenic Ras or c-Myc, the presence of cytokines such as TNFand PDGF, or the interaction of integrins with the extracellular matrix (Figure 21.9). The mechanism by which the ubiquinone pathway is activated remains to be elucidated.37,38
Superoxide is rapidly converted (dismutated) into molecular oxygen (O2) and peroxide (O22 ), which as H2O2, is converted by catalase into oxygen and water (Figure 21.8). The ROS, in particular H2O2, survive long enough to modify the active-site cysteine residue of tyrosine phosphatases.
There have been a number of claims for a role of ROS, in particular H2O2, as intracellular messengers that regulate protein phosphorylation and gene expression. An early hint was provided by the observation that H2O2 can mimic the stimulatory effect of insulin on glucose transport and lipid synthesis in adipocytes.39,40 Later it was found that O2 can stimulate members of the MAP kinase family41 and that blunting the spike of H2O2 (using catalase or the antioxidant N-acetylcysteine) obscures a number of signal transduction events such as phosphorylation and activation of MAP
kinase in response to PDGF. The cells also fail to enter the S-phase of the cell cycle and lose their response to chemotactic stimuli.34
652

Protein Dephosphorylation and Protein Phosphorylation
Further evidence for a second messenger role of ROS came from experiments that showed that EGF-mediated autophosphorylation of its receptor and
the subsequent phosphorylation of receptor substrates can be can be upregulated by the addition of H2O2 to the culture medium. The enhanced phosphorylation can be prevented by intracellular microinjection of catalase. Several studies have demonstrated an inhibitory effect of H2O2 (at micromolar concentrations) on various tyrosine phosphatases, both membrane bound and cytosolic.42–45
Striking evidence for the role of NADPH oxidase in cellular signalling came from the finding that over-expression of NOX1 in NIH3T3 cells produces particularly aggressive tumours following injection into nude (athymic) mice. Sustained and elevated levels of hydrogen peroxide are required to maintain the transformed state.46,47
It is now widely accepted that ROS that are transiently generated in cells in response to extracellular stimuli, and which act on effector systems, can be regarded as second messengers. Oxidative modification of catalytic-site cysteine residues resembles the mode of action of phosphorylation (Figure 21.8). rPTK-induced generation of ROS causes prolonged inactivation of
tyrosine phosphatases and so maintains the phosphorylated (and activated) state of the receptor (positive feedback loop) (Figure 21.9). Their brief generation time and the presence of glutathione (GSH) ensure that over a period of about a few minutes the phosphatases return to their reduced state, capable once again of dephosphorylating (and inactivating) the receptor.48–50
SHP-1 and SHP-2
The SHP phosphatases comprise a subfamily of cytosolic PTPs having two N-terminal SH2 domains. They bind to tyrosine phosphorylated growth factor receptors (EGFR, FGFR, HGFR, PDGFR), cytokine receptors (IFNAR1, IL-R, TNFR1), scaffolding adaptors (IRS, DOS/Gab, FRS proteins) and immune inhibitory receptors (such as Fc RIIB (CD32) and KIR) that contain an ITIM motif).51 The C-termini of SHP-1 and -2 contain two phosphorylation sites which are targets of the PDGF receptor or cytoplasmic kinases such as JAK or
TYK2. When phosphorylated these tyrosines act as docking sites for other SH2 or PTB domain containing proteins (e.g. Grb2, see Figure 21.11).
There are two vertebrate SHP genes, SHP-1 and SHP-2, and invertebrate orthologues such as Csw (corkscrew) in Drosophila and Ptp-2 in C. elegans.52 SHP-1 is mainly (but not exclusively) expressed in haematopoietic cells; SHP-2 is ubiquitously expressed. Despite the general similarity of SHP-1 and SHP-2, their functions are quite distinct. Whereas SHP-1 is generally inhibitory, SHP-2 acts as a stimulator of the Ras–MAPK pathway.
653
