
- •Protein tyrosine phosphatases
- •Cytosolic PTPs
- •Transmembrane receptor-like PTPs
- •Tyrosine specificity and catalytic mechanism
- •PTPs in signal transduction
- •PTP1B, diabetes, and obesity
- •PTP1B as a possible therapeutic target for the treatment of type 2 diabetes and obesity
- •Redox regulation of PTP1B: reactive oxygen species as second messengers
- •Regulation of SHP-1 and -2
- •SHP-1, JAKs, and STAT5
- •SHP-2 and the Ras–MAP kinase pathway
- •Insight through the Noonan syndrome
- •Density enhanced PTP (DEP1)
- •CD45 and the regulation of immune cell function
- •Regulating receptor PTPs
- •Dual specificity phosphatases
- •Regulation of MAP kinases by dual-specificity protein phosphatases (DS-MKP)
- •Physiological role of the dual-specificity MAP kinase phosphatases
- •Dual-specificity phosphatases in development
- •PTEN, a dual-specificity phosphatase for phosphatidyl inositol lipids
- •Serine/threonine phosphatases
- •Classification of the serine/threonine phosphatases
- •Regulation of PPPs
- •Phosphorylation of the catalytic subunits
- •Regulation by intramolecular domain interaction
- •Regulatory subunits of PP1
- •Inhibitors of PP1, PP2A, PP4, and PP5
- •PP1 in the regulation of glycogen metabolism
- •Regulation of glycogen metabolism: muscle
- •Regulation of glycogen metabolism: liver
- •PP2B (calcineurin)
- •Dephosphorylation of NFAT: immunophilins show the way
- •References

Protein Dephosphorylation and Protein Phosphorylation
regulates the activity of PKB in the nucleus is not yet clear. The importance of all this has been highlighted by the finding that the mutation K289E occurs in some cases of Cowden syndrome, and as a consequence PTEN fails to enter the nucleus. Not only this, but failure of nuclear localization also renders PTEN more vulnerable to polyubiquitylation, resulting in its recognition by the proteasome and leading to its destruction (Figure 21.23).
Serine/threonine phosphatases
While all the PTPs are monomeric and their differences are set by their domain structures, the majority of the serine/threonine phosphatases are oligomers and characterized by their association with targeting subunits. These
direct them to particular locations, so restricting their action to a limited range of substrates. The first serine/threonine phosphatase recognized, PP1c, inactivates glycogen phosphorylase.123,124 Other serine/threonine phosphatases, initially classified into four groups PP1, PP2A, PP2B, and PP2C, have since been identified (Table 21.3). Because they are relatively nonspecific in their action, it was thought that this small number of enzymes would be sufficient to balance the effects of the numerous kinases.125 Some functional diversity then became apparent with the discovery of inhibitors
Table 21.3 Classification of the S/T phosphatase catalytic (C) subunits
Name |
Gene name |
|
|
|
|
PPP family |
|
|
|
|
|
PP1 , - , - , - |
PPP1CA, PPP1CB, PPP1CC, PPP1CD |
PP1c is the catalytic subunit of PP1 |
|
|
|
PP2A , - |
PPP2CA, PPP2CB |
plus subunit A-a or A-b |
|
|
|
PP2B ,- , - |
PPP3CA, PPP3CB, PPP3CC |
plus subunit calcineurin B (and calmodulin) |
|
|
|
PP4 |
PPP4C |
PPP4R1 |
|
|
|
PP5 (Ppt1) |
PPP5C |
|
|
|
|
PP6 |
PPP6C |
|
|
|
|
PPM family |
|
|
|
|
|
PP2C ,- |
PPM1A, PPM1B, PPM1D, PPM1F, PPM1G, |
monomer, Mg2 -dependent |
|
PPM1K, PPM1L, PPM1M, ILKAP |
|
|
|
|
PDP |
PPM2C |
mitochondrial pyruvate dehydrogenase |
|
|
phosphatase |
The three-letter abbreviations adhere to the conventions of the human genome nomenclature in the specification of a family. PPP indicates PhosphoProtein Phosphatase, PPM activation by magnesium.
671
Signal Transduction
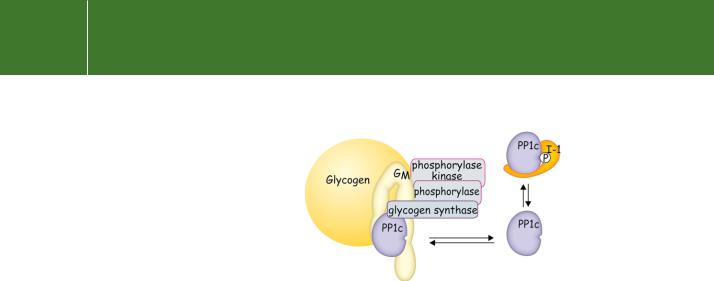
FIG 21.24 Serine/threonine phosphatases, targeting subunits, and inhibitor proteins. PP1c, involved in glycogen metabolism, never exists in a free soluble state. It is bound either to its regulatory subunit GM, associated with glycogen (forming the enzyme complex PP1G), or it is bound to the inhibitory subunit I-1, which prevents uncontrolled dephosphorylation of other substrates.
that affect a restricted range of phosphatases. For example, ciclosporin A, widely used as an immunosuppressive agent after organ transplantation, is a selective inhibitor of PP2B (calcineurin) (page 683), and okadaic acid, a tumour promoter, is a potent inhibitor of PP1 and PP2A (see page 679).
Real diversity came to light when it was realized that the purified activities represent only the catalytic subunits, but that in the cellular context they are coupled with targeting or regulatory subunits. The subcellular distributions, substrate selectivities, and catalytic activities are largely determined by these regulatory subunits. For example, PP1 is active against a wide range of peptide and protein substrates and so its activity must be carefully limited. Under normal conditions, it is coupled with a G subunit (GM in the case of muscle) which has very high affinity for glycogen (Kapp 6 nM) (Figure 21.24). This ensures that its free form is kept at vanishingly low levels. In the situation of severe glycogen depletion or upon detachment due to phosphorylation of GM, soluble inhibitor proteins (inhibitor-1) ensure that its concentration
in the cytosol remains low. In these ways, PP1 is prevented from acting as a loose cannon, randomly dephosphorylating any phosphoprotein that might come in range. The association with particular targeting proteins narrows the range of available substrates. The other enzymes of glycogen metabolism – phosphorylase kinase, phosphorylase, and glycogen synthase –
are also tightly bound to glycogen and all three are good substrates for dephosphorylation by PP1c/GM.
Classification of the serine/threonine phosphatases
The serine/threonine phosphatases are classified in two superfamilies, PPP and PPM, listed in Table 21.3.126,127 The domain architectures of some representative members of these families are shown in Figure 21.25. The PPM
672
Protein Dephosphorylation and Protein Phosphorylation
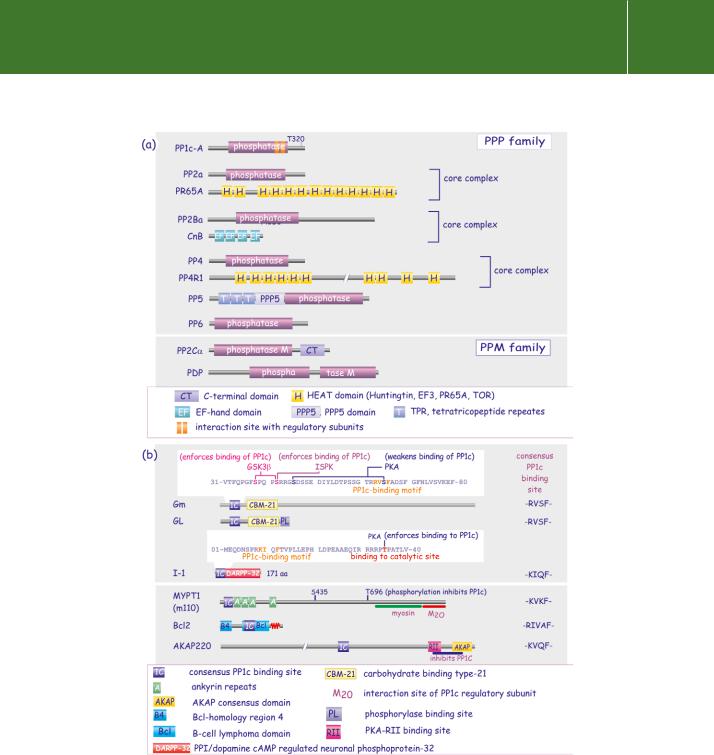
FIG 21.25 (a) Domain architecture of serine/threonine phosphatases (PPP and PPM). With the exception of PP5, they interact with regulatory or targeting subunits. (b) Domain architecture of regulatory subunits discussed in this chapter.
673

Signal Transduction
FIG 21.26 Members of the PPP family share a common catalytic domain structure. (a) The catalytic domain fold consists of a central β-sandwich surrounded on one side by seven α-helices and on the other by a subdomain comprising three α-helices and a three-stranded β-sheet. (b) The catalytic cleft has a Y-shape, with three branches commonly referred to as hydrophobic, acidic and C-terminal grooves. (c) Crystallographic data provide compelling evidence for the role of two metal ions in the catalytic reaction. Most data are consistent with a single step mechanism, employing a metal-activated nucleophilic water molecule or hydroxide ion. (d) The metal coordinating residues (asparagines, aspartates and histidines) are invariant amongst the PPP family members. The position of some of the amino-acids of the signature sequences are shown. (1jk7134).
family includes the Mg2 -dependent PP2C and mitochondrial pyruvate dehydrogenase phosphatase (PDP). The PPP family is characterized by the presence of three invariant amino acid motifs in the catalytic domain and two metal ions required for their activity, though their identity remains controversial, possibly Mn2 Mn2 or Mn2 Fe2 (Figure 21.26). The human genome contains 40 genes encoding the catalytic subunits of the PPP family. Functional diversity is expanded by the expression of splice variants and the existence of a large number of targeting subunits. For instance, PP1c Table 21.4, Figure 21.25 forms complexes with 45 regulatory subunits (bona fide and putative).
674
