
- •Chemotherapy
- •Cytotoxic antibiotics and antimetabolites
- •The purine pathway to chemotherapy
- •Good drugs and bad
- •Combination chemotherapy
- •Alternative targets for cancer therapy: towards a scientific rationale
- •Inhibiting the EGF family of receptor kinases
- •The antibody approach: trastuzumab
- •The tyrosine kinase inhibitor approach
- •Erlotinib
- •Gefitinib
- •Imatinib: chronic myeloid leukaemia and the Bcr-Abl fusion story
- •Development of imatinib, inhibitor of c-Abl
- •Why is treatment of CML successful?
- •Molecular mechanism of inhibition by imatinib
- •Resistance due to mutations at different sites in the Abl moiety of Bcr-Abl
- •Other signal transduction components targeted for therapeutic intervention
- •Towards a different approach in testing cancer drugs?
- •References
Targeting Transduction Pathways for Research and Medical Intervention
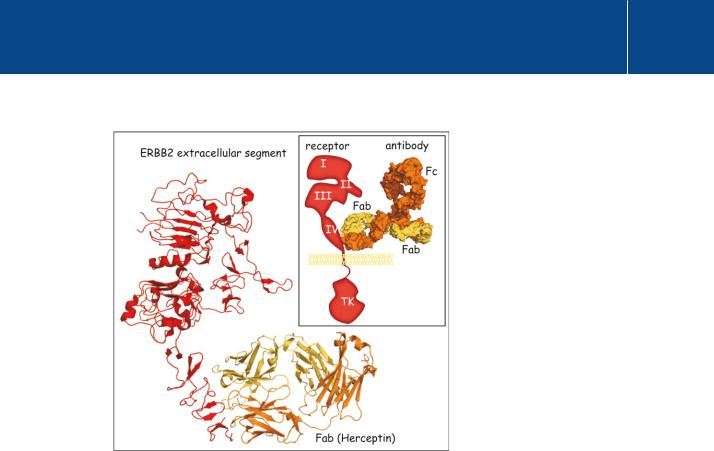
Fig 23.3 The binding of the Fab fragment of trastuzumab to ErbB2.
The main figure shows the extracellular segment of ErbB2 coupled to the monovalent Fab fragment of the monoclonal antibody trastuzumab. The extracellular segment of ErbB2 is composed of four domains (see Figure 12–4, page 321). Domain II (CR1) contains the dimerization finger. Trastuzumab binds to the membraneproximal region of domain IV (CR2) of ErbB2. Although the size of the antibody is impressive, its attachment appears not to prevent receptor dimerization (1n8z32).
The tyrosine kinase inhibitor approach
Most inhibitors of the EGF receptor family of tyrosine kinases are reversible, having high affinity for ATP (of the order of 10 9 mol L 1). Some recently developed compounds are irreversible, forming a covalent bond with residue C773 which is unique for the EGF receptors.
Erlotinib
One such compound is erlotinib (Tarceva). It is used as a single-agent treatment for patients having locally advanced or metastatic non-small cell lung cancer, after failure of at least one prior chemotherapy regimen. It has been approved for treatment of metastatic pancreatic cancers, in combination with the standard chemotherapeutic compound gemcitabine (an antimetabolite).
745

Signal Transduction
Gefitinib
Gefitinib (Iressa) is another EGF-receptor inhibitor. Its structure resembles that of erlotinib, but its future as a drug to fight cancer is less certain. In cell proliferation assays and as an inhibitor of receptor phosphorylation it acts at nanomolar concentrations. Oral administration causes inhibition of tumour growth of mouse xenografts. However, it has a cytostatic, not a cytotoxic effect: withdrawal of the drug after 100 days allowed almost immediate relapse. Initial trials with patients having locally advanced or metastatic non-small-cell lung cancer, which failed to respond to both platinum-based (polymerase inhibitor) and docetaxel (microtubule inhibitor) chemotherapies, appeared promising. Unfortunately, when tested in a large-scale randomized study erlotinib proved to have little effect on survival.
Imatinib: chronic myeloid leukaemia and the Bcr-Abl fusion story
Chronic myeloid leukaemia (CML) is a rare condition in which 96% of the patients who are in the chronic phase carry the same chromosomal abnormality. This is a somatic translocation, t(9:22), between the long arms of chromosome
9 (paternally derived) and chromosome 22 (maternally derived) that gives rise to one very small‘Philadelphia’chromosome (Ph) and one abnormally long chromosome (Figure 23.4). This translocation has been detected in
haematopoietic stem (HSC) cells of both myeloid and erythroid lineages, but it is only the myeloid cells that proliferate during the chronic phase of the disease.35
The t(9:22) translocation gives rise to a deregulated fusion protein, for instance p210Bcr-Abl, that comprises a number of exons of Bcr and all (excepting the first alternatively spliced exons Ia or Ib) of c-Abl (Figure 23.5). The normal c-abl gene codes for a predominantly nuclear tyrosine protein kinase that possesses a number of protein interaction domains (SH3, SH2, DNA, and F-actin binding).
Fig 23.4 Origin of Bcr-Abl.
The fusion protein Bcr-Abl arises by a translocation between chromosomes 9 and 22. The breakage of chromosome 22 occurs at a number of points in the Bcr gene giving rise to different fusion products (190 kDa, 210 kDa, or 230 kDa) associated with various pathologies. Bcr-Abl p210 (see Figure 23.5) is associated with chronic myelogenous leukemia (CML).
746

Targeting Transduction Pathways for Research and Medical Intervention
The Bcr gene also codes for a multidomain protein having catalytic activities (RhoGAP and RhoGEF and a protein/lipid interaction site (PH). The fusion product is a deregulated tyrosine kinase, localized in the cytosol that causes both the aberrant proliferation and the lack of terminal differentiation of the myeloid cells in chronic myeloid leukaemia. Other forms of Bcr-Abl, resulting from different breakpoints in the Bcr gene, are also encountered in various human leukaemias.
The Bcr gene derives its name because it is located at a site of frequent chromosomal breakage, the breakpoint cluster region. Bcr can break at different sites, but the major breakpoints are situated after exons 13 or 14. A minor breakage occurs after exons 1 or 19. The 210 kDa chimeric Bcr-Abl
protein, present in 95% of CML patients, is the product of breakage either after exon 13 (giving rise to b2a2 fusion product) or 14 (giving rise to b3a2 fusion product).
Fig 23.5 Domain architecture of Bcr, Abl and the Bcr-Abl fusion protein.
c-Abl is a cytosolic tyrosine kinase but, unlike Src, it lacks the N-terminal phosphotyrosine. Instead, it has a cap region (and in the case c-Abl1b, a myristoyl chain), which maintain the kinase in a locked inactive conformation. Bcr tends to break at different points, each associated with a different leukaemia (ALL, CML, or N-CML). The chimeric Bcr-Abl protein comprises the N-terminal segment of Bcr attached to an almost intact kinase, though lacking the cap region (and the associated myristoyl chain). The consequences of the fusion are described in Figure 23.7.
747

Signal Transduction
Similar to the Src-like tyrosine kinases, the SH2 and SH3 domains of the nontransforming c-Abl clamp the catalytic domain in an inactive state, referred to as the‘latched conformation’. However, unlike Src, c-Abl lacks the C-terminal tyrosine that imposes the SH2-SH3 clamp when phosphorylated . In c-Abl, this role is played by the N-terminal Cap region with a myristoyl attachment (figure 23.6).36 Under physiological conditions, the clamp is relieved by proteins that interact with either the myristoyl attachment, the SH2 domain (amongst others, c-Jun, CAS, Cbl, or EphB2) or the SH3 domain (amongst others, Abi1, SHIP1, Nck, paxillin, or Cbl). Full activation then occurs through phosphorylation in the
SH2-kinase linker (Y245, which prevents interaction of SH3 with the linker region) and in the activation segment (Y412, which facilitates access of substrate). These phosphorylations are thought to occur through transphosphorylation (c-Abls phosphorylating each other). Members of the Src-family of protein kinases are other possible candidates that might bestow full catalytic competence on c-Abl.
There are several reasons why the behaviour of the fusion protein Bcr-Abl differs from that of c-Abl (for a review, see Wong and Witte38). For instance, Bcr-Abl lacks the Cap domain and the myristoyl attachment, and the Bcr segment of the fusion protein interacts with the SH2 domain of Abl. These all act to prevent the formation of the latched conformation (Figure 23.6 and 23.7). Importantly, unlike c-Abl, Bcr-Abl is a cytosolic protein and must interact with quite different substrates. The presence of the actin-binding domain in
Fig 23.6 Structure of c-Abl.
(a) c-Abl in its closed conformation, with SH3 and SH2 domains clamping the kinase domain in an inactive state. Essential in this is the cap region, with myristate attached at the C-terminal part of the kinase domain. This interaction creates binding sites between the kinase and SH2 domains. Stacking of pY158 and pY361 is important here. The close apposition of SH3 causes interaction with proline residues in the linker region that bridges the SH2 with the kinase domain (P249 and P242). This interaction is prevented by phosphorylation at Y245. (b) Imatinib/Gleevec occupies the ATP binding pocket and holds the activation segment in a lower position. As a consequence, productive phosphorylation is, of course, out of the question. SH2 and SH3 domains are not shown (1opk,37 1opj37).
748
Targeting Transduction Pathways for Research and Medical Intervention
Bcr may also contribute to specific subcellular localizations, thereby offering substrates that c-Abl would never otherwise encounter.
The Bcr-Abl fusion protein has profound effects on cell survival and proliferation. The amount of RasGTP is greatly expanded and the PI 3-kinase pathway is also affected. However, transformation is prevented if dominant negative forms of Ras or PKB (or its inhibitor LY29002) are present. Also,
the phosphatidyl inositol 5-phosphatase SHP-1 is down-regulated and the interferon-receptor STAT pathway is inhibited.
The requirement for continuous expression of Bcr-Abl in CML cells has been demonstrated in a mouse model of the disease. Here, expression of Bcr-Abl was controlled by a tetracycline sensitive promoter so that removal of the tetracycline aborted the expression of the fusion protein. When this was done, there was a remission of the leukaemia.39
Figure 23.7 outlines modifications that alter the activation state of c-Abl.
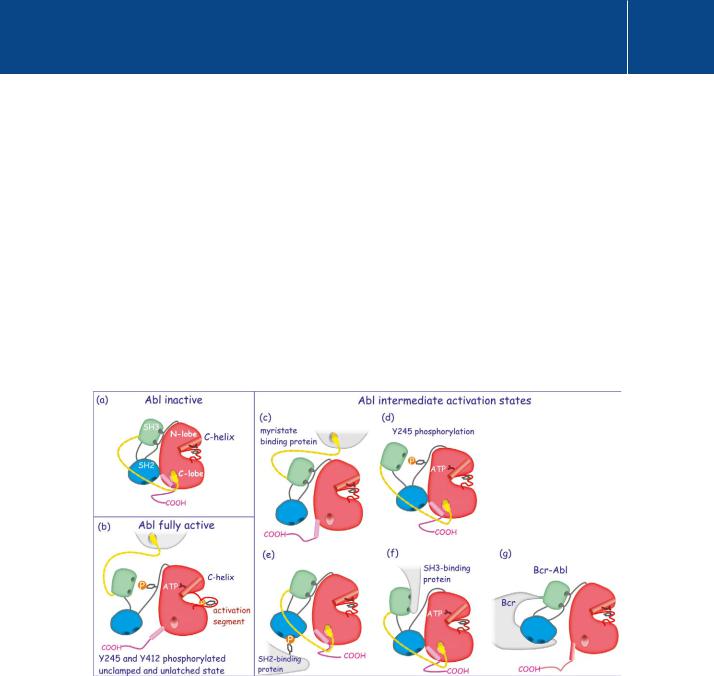
Fig 23.7 Regulation of c-Abl.
Regulation of c-Abl. c-Abl has many different activation states. (a) and (b) represent the inactive and fully active states. Intermediate states, which have limited kinase activity, are represented in panels (c) – (f). (a) Latched conformation, the kinase domain clamped in an inactive state. (b) Displacement of myristate (yellow) from the C-lobe, detachment of the SH3 domain by phosphorylation of Y245 and reorganisation of the activation segment
by phosphorylation of Y412 render c-Abl fully competent. (c) Partial activation through displacement of myristate. (d) Partial activation through phosphorylation of the linker region (Y245). (e) Partial activation through displacement of the SH2 domain by a phosphotyrosine-containing protein. (f) Partial activation through displacement of SH3 by a proline rich sequence. (g) The fusion protein Bcr-Abl is also in an intermediate activation state. It lacks the Cap region (together with its myristate chain) and the Bcr moiety binds and holds the SH2 domain away from the kinase domain. Full activation then requires only phosphorylation of Y245 and Y412.
749
