
Kluwer - Handbook of Biomedical Image Analysis Vol
.1.pdf124 |
Rakesh Sharma and Avdhesh Sharma |
3.1.1.4Factors Influencing the Appearance of Flowing Blood on MR Images
The magnetization Mxy, after application of 90◦ nutation pulse in an SE experiment, can be explained as:
Mxy = F{v, a, . . . }H{1 − exp (t/T1)}(exp(−t/T2)) |
(3.7) |
where the expressions in parentheses characterize longitudinal (T1) and transverse (T2) dependent magnetizations and are known as relaxation times. H is proton density. The factor F(v, a, . . .) is flow factor which depends on spin isochromats. Velocity (v), acceleration (a), slice transition, spin phase phenomena, and high-order motion terms are the main variables.
3.1.2 Flow Physical Principles
Let us discuss the basic flow patterns in blood vessels and related MRA signal magnitudes. Mathematical models of laminar and plug flow for signals from spin echo pulses are well established. Flow causes a physical displacement of spins between successive excitations. This time-of-flight effect leads to different series of RF pulses producing different echo amplitudes for a number of different spin populations. The fraction volume of each population can be expressed as a function of the interpulse interval length during which each population enters the slice. So, the total signal is the sum of the echo amplitudes from each spin population. Different pulse sequences generate image signal intensity (SI) as a function of velocity, TR, T I, TE and slice thickness.
Fractional volume segments. The cylindrical volume of the vessel cut by a slice is equal to fraction of flowing blood volume of imaging (VOI).
MRA image signal. The evolution of magnetization for each spin population can be described by Bloch equations. For simplicity, magnetization may be expressed for transforming to a reference rotating frame at Larmor frequency (−γ H0) according to Bloch equations as follows:
Mx(t) = M0x exp(−t/T2) |
(3.8) |
My(t) = M0y exp(−t/T2) |
(3.9) |
Mz(t) = M0[1 − exp(t−t/T1)] + M0z exp(−t/ T1) |
(3.10) |
Advances in Magnetic Resonance Angiography |
125 |
where M0x, M0y, and M0z are the values of Mx, My, and Mz at t = 0, and M0 is the steady-state magnetization in the z direction. This condition is good for spins rotating in the transverse plane aligning through the x axis at M0y = 0.
For fundamental understanding for the signal generation, we describe different signal intensities of main pulse sequences as follows:
90◦ selective − TE/2 − 180◦: S = M0exp(−TE/T2) 180◦ − (TR − TE/2) − 90◦ − TE/2 − 180◦:
S= M0{1 − 2exp[−(TR − TE/2)/T1]}exp(−TE/T2) 90◦ − TE/2 − 180◦ − (TR − TE/2) − 90◦ − TE/2 − 180◦:
S= M0{1 − 2exp[−(TR − TE/2)/T1] + exp(−TR/T1)}exp(−TE/2)
The complete expression for the echo signal has four separate velocity boundary conditions:
Plug flow signal will be:
SV (TR − TE/2)/D + SVTE/(2D) + S[1 − V (TR + TE/2)D]
if 0 < V < D/(TR + TE/2)
SV (TR − TE/2)/D + S(1 − VTR/D) if D/TR + TE/2) < V < (TR
S[1 − V TE/(2D) if D/TR < V < D/(TE/2) 0 if V > D/(TE/2)
Laminar flow signal will be:
Sa[Vm(TR − TE/2)/(2D)] + Sb VmTE/(4D) + Sc[1 − Vm(TR + TE/2)/2D)]
if 0 < Vm < D/(TR + TE/2)
Sa[Vm(TR − TE/2)/(2D)] + Sb{1 − D/2Vm(TR + TE/2)] − VmTR/(2D)}
+ Sc D/[2Vm(TR + TE/2)] if D/(TR + TE/2) < Vm < D/TR
Sa[1 − D/(2VmTR) − VmTE/4D)] + Sb{D/(2VmTR) − D/[2Vm(TR + TE/2)]}
+ Sc D/[2Vm(TR + TE/2)] if D/(TR) < Vm < D/(TE/2)
Sa[D/ VmTE) − D/2Vm(TR)] + Sb{D/(2VmTR) − D/[2Vm(TR + TE/2)]}
+ Sc D/[2Vm(TR + TE/2)] |
if Vm > D/(TE/2) |
|||||||
90◦ selective |
− |
TI |
− |
90◦ selective |
− |
TE/2 |
− |
180◦ selective pulse: |
|
|
|
|
|||||
a.90◦−TE/2−180◦: Sa = M0 exp (TE/T2)
b.90◦−TI−90◦−TE/2−180◦: Sb = M0[1 − exp(−TI/T1] exp(−TE/T2)
126 |
Rakesh Sharma and Avdhesh Sharma |
3.1.2.1 Slice-Transition Phenomenon
It is known as the time-of-flight effect. The time-of-flight effect occurs whenever a vessel takes course in part perpendicular to the imaging plane. During this course, substantial amount of blood volume is replaced within the imaged slice for several hundred milliseconds. Different pulse sequences capture these events of blood volume turnover in selected slices and voxels of the circulatory system. For example, images are captured in several hundred milliseconds by spin echo (SE) pulse sequence and a few tens of milliseconds in GRE imaging. Normally, the flow direction is selected perpendicular to the imaged slice axis in the xy plane. The flow is chosen along the z direction. Time-of-flight depends upon the slice thickness, position of slices in stack region of interest, multislice acquisition order, flow velocity, and flow direction of spin isochromats. Assume a volume element or voxel that moves with constant velocity V0. If it moves a distance z0 during time t, we can write
V0 = z0/t.
Two types of slice-transition phenomenon can occur. In the first case, blood moves so fast that it leaves the slice between the 90◦ nutation pulse and the 180◦ refocusing pulse in an SE sequence. It results in signal loss and has been termed as high-velocity signal loss. In the second case, the flow velocity is small enough that only a small fraction of blood leaves the slice between the 90◦ and 180◦ pulses in the SE sequence, but a part of the blood is replaced by fully magnetized blood from outside of the imaged volume during the repetition time (TR). It leads to increased intravascular signal and has been termed flow-related enhancement.
3.1.2.1.1 High-Velocity Loss. Spin isochromats leave the imaged slice in an SE sequence before the rephasing pulse for a given echo is applied. These spin isochromats will not contribute to this echo. These spin isochromats continue with their dephasing process or rephrasing. This dephasing process or rephrasing will not be experienced by spin isochromats. In general, the rephasing pulse occurs at time Te, which is equal to TE/2 for the first echo, or (n − 1/2)TE if the nth echo in a multiecho sequence with regular echo spacing is used. Here
Te is equal to TE1 + (TE2 − TE1)/2 if a sequence with two asymmetrical echoes (at times TE1 and TE2) is used. The maximum velocity (Vz) depends on slice
Advances in Magnetic Resonance Angiography |
127 |
thickness (s). The maximum velocity of blood (Vz) above which blood leaves the slice will be more than s/Te. For lower velocities, only a fraction of blood leaves the voxel up to the rephrasing pulse time Te. The fraction of blood (g) will be dependent on maximum velocity and slice thickness as:
g = Vz/s.
Thus, the recorded magnetization decreases linearly with the velocity down to zero. The blood flow may be observed to be very slow. As a result, intravascular signal is seen on first echo, but no longer on second echo. It indicates blood has moved out of the slice during the time interval of TE/2 and 3TE/2. If we know the slice thickness, we can calculate blood flow velocity. The signal loss on the first echo at the rim of the vessel is commonly observed due to spinphase effect in SE sequences. On the contrary, in GRE sequences, slice-selective rephrasing pulse is not applied. So, slice-transition effects are not observed and do not produce any intravascular signal loss. At low blood velocities, only few out of all spin isochromats leave the slice during the time Te. Hence, during the repetition time TR Te applied, some or all spin isochromats can be replaced by still fully magnetized spin isochromats. These spin isochromats represent from outside the imaged volume. In this region, these spin isochromats have not undergone repetitive 90◦ pulse in SE or alpha pulse in GRE sequences. This gives rise to ‘entry slice phenomenon’. We shall discuss entry slice phenomenon in the following section.
3.1.2.1.2 Entry Slice Phenomenon. If T1 TR for blood, spin isochromats cannot fully recover their magnetization along the z axis. If spin isochromats in blood move perpendicular to a stack of slices with velocity v, they are subjected to 90◦ in SE imaging and to an alpha pulse in GRE pulse sequence. Due to flow, they are partly replaced by spin isochromats from outside imaging volume. This outside volume has not been subject to such pulse. So, net magnetization M available for the next 90◦ pulse or alpha pulse is the sum of the magnetization of the remaining spin isochromats. It leads to increased intravascular signal intensity. The temporal dependence of M over time will represent an increase in
M in such a way that the first linear increase will be due to incomplete magnetization recovery. The later phase in the increase in M as a plateau will represent flow-related enhancement. The magnitude of this effect is again dependent on the flow velocity (v), the slice thicknes, and TR. The fraction of blood g in the
128 |
Rakesh Sharma and Avdhesh Sharma |
voxel that is replaced is given by |
|
g1 = vT/s |
(3.11) |
while other one remaining is given by 1 − g2. The sum of the two magnetization components in the vessel is thus
M(TR) = M{(1 − g1)[1 − exp(−TR/T1)] + g2}{(1 − g1)[1 − exp(−TR/T1)]}
(3.12) It represents previous voxel in slice and g2 represents fresh voxel. The flowrelated enhancement is prominent when a significant fraction of blood in a slice is replaced during the time TR. With optimized values for slice thickness, s, and repetition time, TR, in a sequence, such flow velocities, v, are of the order of a few centimeters per second. At TR = 500 msec, s = 0.5 cm, the flow velocity will measure 1 cm/sec. At higher velocities the combination of higher velocity signal loss and flow-related enhancement tends to reduce the intravascular signal intensity.
3.1.2.1.3 Flow-Related Enhancement. During multislice acquisition, it can be operative in several slices of a stack. The spin isochromats moving at the center of a vessel are generally faster than those close to the vessel wall. Therefore, centrally located spin isochromats move deeper onto the stack during the repetition time (TR) than peripherally located ones. Suppose the planes of entry into different slices are separated by a distance q(q slice thickness), then fully magnetized spin isochromats moving with a velocity v will enter the jth slice after a time, t = j · q/v after entering the first slice. Spin isochromats that move a distance j · q after the jth slice and before the ( j + 1)th slice are irradiated with RF pulses. These contribute their full magnetization to the signal measured in the ( j + 1)th slice from outside the stack without being disturbed by RF irradiation. Here slices are acquired in the sequence parallel to flow. The deeper slice in the stack indicates that faster blood flow enhances the signal in that slice. The fast flow causes high-velocity signal loss in SE images. Thus flow-related enhancement cannot be observed in all slices of a stack. Suppose q = 10 mm, TE = 30 msec, and adjacent slices are excited 100 msec apart, the total signal loss will occur for velocities of approximately 65 cm/sec and above according to V = K · s/TE where K and s are slice thickness. Blood moving through the first slice between the refocusing pulse at T = 15 msec and into the
Advances in Magnetic Resonance Angiography |
129 |
next slice before it is excited at t = 100 msec will contribute full magnetization to the second slice. Such spin isochromats must move with a velocity V of at least 1 cm/(100 − 15 msec) = 12 cm/sec, which is lower than 65 cm/sec (velocity needed for total signal loss). Such spin isochromats contribute to signal enhancement in the second slice. In fact, isochromats moving at approximately 24 cm/sec and 36 cm/sec can reach the third and fourth slices, respectively, to contribute to the signal enhancement. However, spin isochromats getting to slices farther into the stack move progressively faster and these isochromats approach the velocities at which high-velocity signal loss occurs. On this basis, it is possible to detect a bright spot of signal with decreased diameter at multiple slices into a stack. On the contrary, if the order of acquisition is reversed in another way to minimize crosstalk between slices, signal enhancement may occur in slices even deeper into the stack. Entry slice effects are principal causes for the high signal intensity of blood vessels on gradient-recalled-echo (GRE) images. This is a result of the fact that the short TR in GRE sequences does not permit the z magnetization to regrow to the values close to its maximum Mz0. It is only a small fraction when the next alpha (α) pulse is applied. If a substantial fraction of blood is replaced during the TR of the sequence, entry slice effects can lead to a very strong signal increase. It results in invisible high-velocity signal loss. It is due to the fact that no slice-selective rephrasing pulse is applied. As a result, intravascular signal will be very bright.
3.1.2.1.4 Slice Transition Effects. These slice transition variations measure the flow in a vessel. The velocity of flowing spins depends upon the distance traveled by the flowing spin isochromats and their travel time. Hence, their velocity may be calculated by dividing these two quantities. Velocity may be measured as the number of excited spins present inside the voxel of interest as a function of time. For this, one way is to apply a slice-selective 90◦ pulse and then to apply a 180◦ rephasing pulse in the slice-displaced phase along the direction of flow at some distance. Any signal measured in this second-slice duration will represent spin isochromats that have been washed-in by the flow in the vessel. This type of method of determination of the flow velocity is advantageous over the spinphase method. In this method, flow sensitization occurs by selecting a read-out slice either proximal or distal to the tagging slice. However, the disadvantage of this approach is that it measures the flow in positive, negative, or in both directions. So, clinically this method is not acceptable.
130 |
Rakesh Sharma and Avdhesh Sharma |
3.1.2.2 Inflow Related Artifacts and Their Suppression
High intravascular signal intensity on GRE images shows the vascular ghost appearance. These ghosts appear due to pulsation artifacts. These pulsation artifacts affect the image quality, if the images are acquired without electrocardiographic (ECG) triggering. These artifacts may be suppressed by the use of phase encoding steps. For this, the principle frequency is selected as superimposed with inspiration rate. It will shift the ghost to the edge of the image. Motion-insensitive pulse sequence is also used to suppress the artifacts. In general, second echo and velocity-insensitive gradients are applied. These methods compensate for high-order motions and thereby minimize the vascular ghosting. Hence, these methods are known as flow-compensated techniques. However, for quantification of vascular features, the spatial-presaturation method is a current practice. This method uses 90◦ RF pulse. The 90◦ RF pulse is applied outside the imaged volume. This imaged volume represents the stack of slices in any region. In any selected region, the inflowing spins may produce entry-slice phenomenon. After applying 90◦ RF pulse, the z magnetization of inflowing spins is completely abolished just before their flow in the imaging volume. This results in no flow-related enhancement effects and the vessels appear black on GRE images. The disadvantage of this method is that it cannot completely suppress signal from stagnant blood imaged with pulse sequences even using the long repetition times (TR).
In order to overcome this problem, MR-projection angiograms of vessels have emerged as an alternative method. In this method, many adjacent thin slices perpendicular to the principal vessel orientation are acquired consecutively. The method is known as 2D Fourier transform gradient recalled echo (2D-FT GRE). As a result of entry-slice phenomenon, the vessels appear very bright in these slices, particularly if they have been acquired using flow compensation to suppress the dephasing. These acquired data are three dimensionally reconstructed using maximal-intensity-projection or surface-rendering methods to generate vascular angiograms. This method is described later in the section on techniques. If presaturation pulse is applied in distal to imaged slice, the signals from veins are suppressed. Similarly, if presaturation pulse is applied in the proximal direction to the imaged slice, it will suppress arterial signals. This method was further refined by the use of two image sequences of the veins and arteries being imaged. It was done by subtracting a flow compensated sequence

Advances in Magnetic Resonance Angiography |
131 |
from the uncompensated sequence or by subtracting a fully presaturated image from a unilaterally presaturated image. The image acquisition in the interleaved fashion will further minimize the motion artifacts.
3.1.2.3 Spin Phase Phenomenon
This effect is based on the motion in a vessel in the direction of magnetic field gradients. It leads to the precession phases different from zero in bulk motion, while the magnitude of the magnetization vector remains unaffected. All of the moving spin isochromats within the voxel experience the same phase change. Interestingly, the moving fluid will have a different phase. Flowing blood gives rise to a velocity profile in a vessel, divided into different voxels. Due to phase change along the vessel wall and surrounding regions, velocity variation is observed due to phase changes either 90◦ or 180◦. It causes considerable signal loss in the voxel at the location of fat tissue.
Suppose a velocity difference of 1 cm/sec within a voxel produces precessional phase changes of approximately 360◦, it will lead to complete signal loss by use of SE sequence with typical gradient values. For slower blood flow, intravascular signal is seen less dephased and is more prominent at the center of the vessel such as accelerated blood flow. With acceleration, the signal loss that results from the dephasing of spin isochromats increases in proportion to the echo number (see Fig. 3.4). For constant velocity motion, this method may be known as even-echo rephrasing or even-echo refocusing for the flow along the
Figure 3.4: Intravoxel spin-phase dispersion due to incoherence is shown near the center of the vessel (point A) for minimal phase dispersion. Point B near the vessel wall encompasses a large range of velocities resulting with intravoxel dephasing and signal loss.
132 |
Rakesh Sharma and Avdhesh Sharma |
direction of a symmetrical gradient field. This is the case of read-out gradient when multiple echoes with constant echo spacing are obtained. In a multiecho sequence obtained in this fashion, echoes occur at all multiple integers of the echo time (TE) such that even echoes correspond to phase change zero for stationary and moving spin isochromats independent of their velocity. The intravascular signal does not show up and results in no dephasing at even echoes. So, the rephrasing phenomenon is dramatic, as vessels without signal on the first echo can have very bright signal on the second echos or even echos. Arteries do have the pulsatile blood flow and experience the velocity and acceleration dephasing effects. During diastole, arterial blood is almost stagnant and leads to high signal intensity. There is complete signal loss during diastole when blood is moving fast in the veins.
Both slice-transition and spin-phase phenomena are responsible simultaneously for the suppression of intravascular signal. In in-plane flow, spin-phase effects must be dominant, whereas for in-flow perpendicular to the imaging plane, slice-transition effects must be prominent. In gradient echo imaging, intraluminal signal loss occurs due to spin-phase phenomenon. It does not show high-velocity signal loss. This intraluminal signal loss results from spatial variation in velocities and accelerations at the site of increased shear stress in the vascular tree such as arterial bifurcation, arterial proximal parts, pulmonary arteries, and venous confluences around cardiac valves. However, dephasing effects and therefore the signal losses on GRE images are pulse sequence dependent.
In general, MR imaging requires the product of the gradient field G and the time to turn-on (tp) to be constant for spatial resolution. So, the dephasing effect on constant velocity is dependent on tp2 and tp3, and shorter TE. In other words, shorter TE and tp will result in less dephasing effect and less signal loss. The SE and GRE pulse sequences are standardized for using dephasing effect in the evaluation of valvular diseases.
3.1.2.4 Turbulence-Induced Signal Loss
On GRE images around vascular stenoses, turbulence-induced signal loss further deteriorates the capacity of this approach in flow quantification in valvular diseases. Spin dephasing and consecutive signal loss make the lesions appear more prominent. The turbulence-induced effects are corrected by flow-compensated
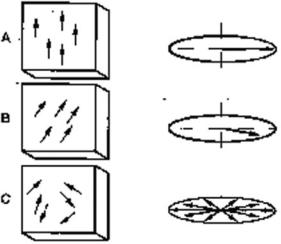
Advances in Magnetic Resonance Angiography |
133 |
Figure 3.5: Velocity-induced phase shifts. Stationary pins do not show any velocity-induced phase shift or intravoxel dephasing (box A). Constant velocity flow throughout voxel results in net phase shift (box B). Turbulence and shear stress within a voxel produce randomly oriented velocity vectors, resulting in a loss of signal.
sequences at short echo times (see Fig. 3.5). These recent advances are described later in this chapter. The velocity distribution inside the voxels is not the result of an intravascular flow profile, but there are multiple small vessels inside a single voxel that take course in all directions. In the case of vessels taking many turns, intravascular velocities and accelerations inside the voxel are due to spatial arrangement rather than the flow profile. Spin isochromats experience destructive interference of isochromat vector components. This results in signal loss and the magnitudes depend upon the cardiac cycle. These signal losses may be avoided by the use of subtraction of flow-sensitive images from the flow-insensitive images to demonstrate tissue perfusion and tissue diffusion.
3.1.2.5 Quantification of Flow by Spin-Phase Effect
Intravascular signals of voxel within the vessel are characterized by the magnitude and phase angle of the magnetization vector. Flow-compensated pulse sequences are sensitive to velocities because the phase angle is a measure of blood flow velocity or acceleration. The phase angle is adjusted below 360◦ for
