
Biomedical EPR Part-B Methodology Instrumentation and Dynamics - Sandra R. Eaton
.pdf302 CANDICE S. KLUG AND JIMMY B. FEIX
Eaton, S. S. and Eaton, G. R. (2000) Determination of Distances Based on  and
and  Effects in Biological Magnetic Resonance (Berliner, L. J., Eaton, S. S., and Eaton, G. R., Eds.) pp 347-381, Kluwer Academic/Plenum Publishers, New York.
Effects in Biological Magnetic Resonance (Berliner, L. J., Eaton, S. S., and Eaton, G. R., Eds.) pp 347-381, Kluwer Academic/Plenum Publishers, New York.
Edwards, T. E., Okonogi, T. M., Robinson, B. H., and Singh, R. J. (2001) Site-Specific Incorporation of Nitroxide Spin-Labels into Internal Sites of the TAR RNA; StructureDependent Dynamics of RNA by EPR Spectroscopy. J. Am. Chem. Soc. 123: 1527-1528.
Fanucci, G. E., Cadieux, N., Piedmont, C. A., Kadner, R. J., and Cafiso, D. S. (2002) Structure and Dynamics of the beta-Barrel of the Membrane Transporter BtuB by SiteDirected Spin Labeling. Biochemistry 41: 11543-11551.
Farahbakhsh, Z. T., Altenbach, C., and Hubbell, W. L. (1992) Spin labeled cysteines as sensors for protein-lipid interaction and conformation in rhodopsin. Photochem. Photobiol. 56: 1019-1033.
Farahbakhsh, Z. T., Hideg, K., and Hubbell, W. L. (1993) Photoactivated conformational changes in rhodopsin: a time-resolved spin label study. Science 262: 1416-1419.
Farrens, D. L., Altenbach, C., Yang, K., Hubbell, W. L., and Khorana, H. G. (1996) Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274: 768-770.
Feix, J. B. and Klug, C. S. (1998) Site-directed spin labeling of membrane proteins and peptide-membrane interactions in Biological Magnetic Resonance, Volume 14: Spin Labeling: The Next Millennium (Berliner, L. J., Ed.) pp 252-281, Plenum Press, New York.
Frazier, A. A., Wisner, M. A., Malmberg, N. J., Victor, K. G., Fanucci, G. E., Nalefski, E. A., Falke, J. J., and Cafiso, D. S. (2002) Membrane orientation and position of the C2 domain from cPLA2 by site-directed spin labeling. Biochemistry 41: 6282-6292.
Froncisz, W. and Hyde, J. S. (1982) The loop-gap resonator: a new microwave lumped circuit ESR sample structure. J. Magn. Reson. 47: 515-521.
Glasgow, B. J., Gasymov, O. K., Abduragimov, A. R., Yusifov, T. N., Altenbach, C., and Hubbell, W. L. (1999) Side chain mobility and ligand interactions of the G strand of tear lipocalins by site-directed spin labeling. Biochemistry 38: 13707-13716.
Grigoryants, V. M., Veselov, A. V., and Scholes, C. P. (2000) Variable velocity liquid flow EPR applied to submillisecond protein folding. Biophys. J. 78: 2702-2708.
Gross, A., Columbus, L., Hideg, K., Altenbach, C., and Hubbell, W. L. (1999) Structure of the KcsA potassium channel from Streptomyces lividans: a site-directed spin labeling study of the second transmembrane segment. Biochemistry 38: 10324-10335.
Gross, A. and Hubbell, W. L. (2002) Identification of protein side chains near the membraneaqueous interface: a site-directed spin labeling study of KcsA. Biochemistry 41: 1123-
1128. |
|
|
|
|
Hanson, P., Millhauser, |
G., Formaggio, F., |
Crisma, |
M., and Toniolo, C. |
(1996) ESR |
Characterization of Hexameric, Helical Peptides Using Double TOAC Spin Labeling. J. |
||||
Am. Chem. Soc. 118: |
7618-7625. |
|
|
|
Hess, J. F., Voss, J. C., |
and FitzGerald, P. G. |
(2002) |
Real-time observation |
of coiled-coil |
domains and subunit assembly in intermediate filaments. J. Biol. Chem. 277: 3551635522.
Hubbell, W. L., Cafiso, D. S., and Altenbach, C. (2000) Identifying conformational changes with site-directed spin labeling. [Review]. Nat. Struct. Biol. 7: 735-739.
Hubbell, W. L., Froncisz, W., and Hyde, J. S. (1987) Continuous and Stopped Flow EPR Spectrometer Based on a Loop Gap Resonator. Rev. Sci. Instr. 58: 1879-1886.
Hubbell, W. L., Gross, A., Langen, R., and Lietzow, M. A. (1998) Recent advances in sitedirected spin labeling of proteins. [Review]. Curr. Opin. Struct. Biol. 8: 649-656.
SDSL: A SURVEY OF BIOLOGICAL APPLICATIONS |
303 |
Hubbell, W. L., Mchaourab, H. S., Altenbach, C., and Lietzow, M. A. (1996) |
Watching |
proteins move using site-directed spin labeling. Structure 4: 779-783. |
|
Hustedt, E. J. and Beth, A. H. (1996) Determination of the orientation of a band 3 affinity spin-label relative to the membrane normal axis of the human erythrocyte. Biochemistry 35: 6944-6954.
Hustedt, E. J. and Beth, A. H. (1999) Nitroxide spin-spin interactions: applications to protein structure and dynamics. [Review]. Annual Review of Biophysics & Biomolecular Structure 28: 129-153.
Hustedt, E. J. and Beth, A. H. (2000) Structural Information from CW-EPR Spectra of Dipolar Coupled Nitroxide Spin Labels in Biological Magnetic Resonance (Berliner, L. J., Eaton, S. S., and Eaton, G. R., Eds.) pp 155-184, Kluwer Academic/Plenum Publishers, New York.
Hustedt, E. J., Smirnov, A. I., Laub, C. F., Cobb, C. E., and Beth, A. H. (1997) Molecular distances from dipolar coupled spin-labels: the global analysis of multifrequency continuous wave electron paramagnetic resonance data. Biophys. J. 72: 1861-1877.
Isas, J. M., Langen, R., Haigler, H. T., and Hubbell, W. L. (2002) Structure and dynamics of a helical hairpin and loop region in annexin 12: a site-directed spin labeling study.
Biochemistry 41: 1464-1473.
Jeschke, G., Pannier, M., and Spiess, H. W. (2000) Double Electron-Electron Resonance: Methodical Advances and Application to Disordered Systems in Biological Magnetic Resonance (Berliner, L. J., Eaton, S. S., and Eaton, G. R., Eds.) pp 493-412, Kluwer Academic/Plenum Publishers, New York.
Karim, C. B., Stamm, J. D., Karim, J., Jones, L. R., and Thomas, D. D. (1998) Cysteine reactivity and oligomeric structures of phospholamban and its mutants. Biochemistry 37: 12074-12081.
Kersten, M. V., Dunn, S. D., Wise, J. G., and Vogel, P. D. (2000) Site-directed spin-labeling of the catalytic sites yields insight into structural changes within the F0F1-ATP synthase of Escherichia coli. Biochemistry 39: 3856-3860.
Kim, C. S., Kweon, D. H., and Shin, Y. K. (2002) Membrane Topologies of Neuronal SNARE Folding Intermediates. Biochemistry 41: 10928-10933.
Klug, C. S., Eaton, S. S., Eaton, G. R., and Feix, J. B. (1998) Ligand-induced conformational change in the ferric enterobactin receptor FepA as studied by site-directed spin labeling and time-domain ESR. Biochemistry 37: 9016-9023.
Klug, C. S. and Feix, J. B. (1998) Guanidine hydrochloride unfolding of a transmembrane beta-strand in FepA using site-directed spin labeling. Protein Sci. 7: 1469-1476.
Klug, C. S., Su, W., and Feix, J. B. (1997) Mapping of the residues involved in a proposed beta-strand located in the ferric enterobactin receptor FepA using site-directed spinlabeling. Biochemistry 36: 13027-13033.
Klug, C. S., Su, W., Liu, J., Klebba, P. E., and Feix, J. B. (1995) Denaturant unfolding of the ferric enterobactin receptor and ligand-induced stabilization studied by site-directed spin labeling. Biochemistry 34: 14230-14236.
Koteiche, H. A., Berengian, A. R., and Mchaourab, H. S. (1998) Identification of protein folding patterns using site-directed spin labeling. Structural characterization of a betasheet and putative substrate binding regions in the conserved domain of alpha A-crystallin.
Biochemistry 37: 12681-12688.
Koteiche, H. A., Narasimhan, C., Runquist, J. A., and Miziorko, H. M. (1995) Utility of a novel spin-labeled nucleotide in investigation of the substrate and effector sites of phosphoribulokinase. Biochemistry 34: 15068-15074.
304 CANDICE S. KLUG AND JIMMY B. FEIX
Kweon, D. H., Kim, C. S., and Shin, Y. K. (2002) The membrane-dipped neuronal SNARE complex: a site-directed spin labeling electron paramagnetic resonance study.
Biochemistry 41: 9264-9268.
Langen, R., Cai, K., Altenbach, C., Khorana, H. G., and Hubbell, W. L. (1999) Structural features of the C-terminal domain of bovine rhodopsin: a site-directed spin-labeling study.
Biochemistry 38: 7918-7924.
Langen, R., Isas, J. M., Hubbell, W. L., and Haigler, H. T. (1998a) A transmembrane form of annexin XII detected by site-directed spin labeling. Proc. Natl. Acad. Sci. USA 95: 1406014065.
Langen, R., Isas, J. M., Luecke, H., Haigler, H. T., and Hubbell, W. L. (1998b) Membranemediated assembly of annexins studied by site-directed spin labeling. J. Biol. Chem. 273: 22453-22457.
Langen, R., Oh, K. J., Cascio, D., and Hubbell, W. L. (2000) Crystal structures of spin labeled T4 lysozyme mutants: implications for the interpretation of EPR spectra in terms of structure. Biochemistry 39: 8396-8405.
Larsen, R. G. and Singel, D. J. (1993) Double electron-electron resonance spin-echo modulation: Spectroscopic measurement of electron spin pair separations in orientationally disordered solids. Journal of Chemical Physics 98: 5134-5146.
Leigh, Jr. J. S. (1970) ESR Rigid-Lattice Line Shape in a System of Two Interacting Spins.
The Journal of Chemical Physics 52: 2608-2612.
Lewis, J. R. and Cafiso, D. S. (1999) Correlation between the free energy of a channelforming voltage-gated peptide and the spontaneous curvature of bilayer lipids.
Biochemistry 38: 5932-5938. |
|
Li, S. C. and Deber, C. M. (1994) |
A measure of helical propensity for amino acids in |
membrane environments. Nat. Struct. Biol. 1: 558. |
|
Lietzow, M. A. and Hubbell, W. L. |
(1998) Site-directed spin labeling of cellular retinol- |
binding protein (CRBP): examination of a b-sheet landscape and its conformational dynamics. Biophys. J. 74: A278.
Lin, Y., Nielsen, R., Murray, D., Hubbell, W. L., Mailer, C., Robinson, B. H., and Gelb, M. H. (1998) Docking phospholipase A2 on membranes using electrostatic potentialmodulated spin relaxation magnetic resonance. Science 279: 1925-1929.
Liu, Y. S., Sompornpisut, P., and Perozo, E. (2001) Structure of the KcsA channel intracellular gate in the open state. Nat. Struct. Biol. 8: 883-887.
Lundberg, K. M., Stenland, C. J., Cohen, F. E., Prusiner, S. B., and Millhauser, G. L. (1997) Kinetics and mechanism of amyloid formation by the prion protein H1 peptide as determined by time-dependent ESR. Chem. Biol. 4: 345-355.
Macosko, J. C., Kim, C. H., and Shin, Y. K. (1997) The membrane topology of the fusion peptide region of influenza hemagglutinin determined by spin-labeling EPR. J. Mol. Biol. 267: 1139-1148.
Macosko, J. C., Pio, M. S., Tinoco, I., Jr., and Shin, Y. K. (1999) A novel 5 displacement spin-labeling technique for electron paramagnetic resonance spectroscopy of RNA. Rna-A Publication of the Rna Society 5: 1158-1166.
Mangels, M. L., Harper, A. C., Smirnov, A. I., Howard, K. P., and Lorigan, G. A. (2001) Investigating magnetically aligned phospholipid bilayers with EPR spectroscopy at 94 GHz. J. Magn. Reson. 151: 253-259.
Marchetto, R., Schreier, S., and Nakaie, C. R. (1993) A Novel Spin-Labeled Amino Acid Derivative for Use in Peptide Synthesis: (9-Fluorenylmethyloxycarbonyl)-2,2,6,6- tetramethylpiperidine-N-oxyl-4-amino-carboxylic Acid. J. Am. Chem. Soc. 115: 1104211043.
SDSL: A SURVEY OF BIOLOGICAL APPLICATIONS |
305 |
Margittai, M., Fasshauer, D., Pabst, S., Jahn, R., and Langen, R. (2001) |
Homoand |
heterooligomeric SNARE complexes studied by site-directed spin labeling. J. Biol. Chem. 276: 13169-13177.
Matthews, B. W. (1995) Studies on protein stability with T4 lysozyme. [Review]. Adv. Prot. Chem. 46: 249-278.
Mchaourab, H. S., Hyde, J. S., and Feix, J. B. (1994) Binding and state of aggregation of spin-labeled cecropin AD in phospholipid bilayers: effects of surface charge and fatty acyl chain length. Biochemistry 33: 6691-6699.
Mchaourab, H. S., Kalai, T., Hideg, K., and Hubbell, W. L. (1999) Motion of spin-labeled side chains in T4 lysozyme: effect of side chain structure. Biochemistry 38: 2947-2955.
Mchaourab, H. S., Lietzow, M. A., Hideg, K., and Hubbeli, W. L. (1996) Motion of spinlabeled side chains in T4 lysozyme. Correlation with protein structure and dynamics.
Biochemistry 35: 7692-7704.
Mchaourab, H. S., Oh, K. J., Fang, C. J., and Hubbell, W. L. (1997) Conformation of T4 lysozyme in solution. Hinge-bending motion and the substrate-induced conformational transition studied by site-directed spin labeling. Biochemistry 36: 307-316.
Mchaourab, H. S. and Perozo, E. (2000) Determination of Protein Folds and Conformational Dynamics Using Spin Labeling EPR Spectroscopy in Biological Magnetic Resonance, Volume 19 (Berliner, L. J., Eaton, S. S., and Eaton, G. R., Eds.) pp 185-247, Kluwer Academic/Plenum Publishers, New York.
McNulty, J., Thompson, D., Carrasco, M., and Millhauser, G. (2002) Dap-SL: a new sitedirected nitroxide spin labeling approach for determining structure and motions in synthesized peptides and proteins. FEBS Lett. 529: 243.
McNulty, J. C. and Millhauser, G. L. (2000) TOAC: The Rigid Nitroxide Side Chain in Biological Magnetic Resonance (Berliner, L. J., Eaton, S. S., and Eaton, G. R., Eds.) pp 277-307, Kluwer Academic/Plenum Publishers, New York.
McNulty, J. C., Silapie, J. L., Carnevali, M., Farrar, C. T., Griffin, R. G., Formaggio, F., Crisma, M., Toniolo, C., and Millhauser, G. L. (2000) Electron spin resonance of TOAC labeled peptides: folding transitions and high frequency spectroscopy. Biopolymers 55: 479-485.
Medkova, M., Preininger, A. M., Yu, N.-J., Hubbell, W. L., and Hamm, H. E. (2002) Conformational Changes in the Amino-Terminal Helix of the G-Protein ail Following Dissociation From Gbg Subunit and Activation. Biochemistry 41: 9962-9972.
Merianos, H. J., Cadieux, N., Lin, C. H., Kadner, R. J., and Cafiso, D. S. (2000) Substrateinduced exposure of an energy-coupling motif of a membrane transporter. Nat. Struct. Biol. 7: 205-209.
Miller, T. R., Alley, S. C., Reese, A. W., Solomon, M. S., McCallister, W. V., Mailer, C., Robinson, B. H., and Hopkins, P. B. (1995) A Probe for Sequence-Dependent Nucleic Acid Dynamics. J. Am. Chem. Soc. 117: 9377-9378.
Mollaaghababa, R., Steinhoff, H. J., Hubbell, W. L., and Khorana, H. G. (2000) Timeresolved site-directed spin-labeling studies of bacteriorhodopsin: loop-specific conformational changes in M. Biochemistry 39: 1120-1127.
Nordio, P. L. (1976) General magnetic resonance theory in Spin Labeling Theory and Applications (Berliner, L. J., Ed.) pp 5-52, Academic Press, New York.
Oh, K. J., Zhan, H., Cui, C., Hideg, K., Collier, R. J., and Hubbell, W. L. (1996) Organization of diphtheria toxin T domain in bilayers: a site-directed spin labeling study.
Science 273:810-812.
Pake, G. E. (1948) Nuclear Resonance Absorption in Hydrated Crystals: Fine Structure of the Proton Line. Journal of Chemical Physics 16: 327-336.
306 |
CANDICE S. KLUG AND JIMMY B. FEIX |
Pannier, M., Veit, S., Godt, A., Jeschke, G., and Spiess, H. W. (2000) Dead-time free measurement of dipole-dipole interactions between electron spins. J. Magn. Reson. 142: 331-340.
Panse, V. G., Beena, K., Philipp, R., Trommer, W. E., Vogel, P. D., and Varadarajan, R. (2001) Electron spin resonance and fluorescence studies of the bound-state conformation of a model protein substrate to the chaperone SecB. J. Biol. Chem. 276: 33681-33688.
Perozo, E., Cortes, D. M., and Cuello, L. G. (1998) Three-dimensional architecture and gating mechanism of a K+ channel studied by EPR spectroscopy. Nat. Struct. Biol. 5: 459469.
Perozo, E., Cortes, D. M., and Cuello, L. G. (1999) Structural rearrangements underlying K+-channel activation gating. Science 285: 73-78.
Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A., and Martinac, B. (2002a) Open channel structure of MscL and the gating mechanism of mechanosensitive channels.
Nature 418: 942-948.
Perozo, E., Kloda, A., Cortes, D. M., and Martinac, B. (2002b) Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 9: 696-703.
Pfannebecker, V., Klos, H., Hubrich, M., Volkmer, T., Heuer, A., Wiesner, U., and Spiess, H. W. (1996) Determination of End-to-End Distances in Oligomers by Pulsed EPR. J. Phys. Chem. 100: 13428-13532.
Qin, P. Z., Butcher, S. E., Feigon, J., and Hubbell, W. L. (2001) Quantitative analysis of the isolated GAAA tetraloop/receptor interaction in solution: a site-directed spin labeling study. Biochemistry 40: 6929-6936.
Qin, Z. and Cafiso, D. S. (1996) Membrane structure of protein kinase C and calmodulin binding domain of myristoylated alanine rich C kinase substrate determined by sitedirected spin labeling. Biochemistry 35: 2917-2925.
Rabenstein, M. D. and Shin, Y. K. (1995) Determination of the distance between two spin labels attached to a macromolecule. Proc. Natl. Acad. Sci. USA 92: 8239-8243.
Rabenstein, M. D. and Shin, Y. K. (1996) HIV-1 gp41 tertiary structure studied by EPR spectroscopy. Biochemistry 35: 13922-13928.
Ramos, A. and Varani, G. (1998) A New Method to Detect Long-Range Protein-RNA Contacts: NMR Detection of Electron-Proton Relaxation Induced by Nitroxide SpinLabeled RNA. J. Am. Chem.Soc. 120: 10992-10993.
Rauch, M. E., Ferguson, C. G., Prestwich, G. D., and Cafiso, D. S. (2002) Myristoylated alanine-rich C kinase substrate (MARCKS) sequesters spin-labeled phosphatidylinositol 4,5-bisphosphate in lipid bilayers. J. Biol. Chem. 277: 14068-14076.
Resek, J. F., Farahbakhsh, Z. T., Hubbell, W. L., and Khorana, H. G. (1993) Formation of the meta II photointermediate is accompanied by conformational changes in the cytoplasmic surface of rhodopsin. Biochemistry 32: 12025-12032.
Rink, T., Riesle, J., Oesterhelt, D., Gerwert, K., and Steinhoff, H. J. (1997) Spin-labeling studies of the conformational changes in the vicinity of D36, D38, T46, and E161 of bacteriorhodopsin during the photocycle. Biophys. J. 73: 983-993.
Russell, C. J., Thorgeirsson, T. E., and Shin, Y. K. (1996) Temperature dependence of polypeptide partitioning between water and phospholipid bilayers. Biochemistry 35: 95269532.
Russell, C. J., Thorgeirsson, T. E., and Shin, Y. K. (1999) The membrane affinities of the aliphatic amino acid side chains in an alpha-helical context are independent of membrane immersion depth. Biochemistry 38: 337-346.
Salwinski, L. and Hubbell, W. L. (1999) Structure in the channel forming domain of colicin E1 bound to membranes: the 402-424 sequence. Protein Sci. 8: 562-572.
SDSL: A SURVEY OF BIOLOGICAL APPLICATIONS |
307 |
Serag, A. A., Altenbach, C., Gingery, M., Hubbell, W. L., and Yeates, T. O. (2001) Identification of a subunit interface in transthyretin amyloid fibrils: evidence for selfassembly from oligomeric building blocks. Biochemistry 40: 9089-9096.
Serag, A. A., Altenbach, C., Gingery, M., Hubbell, W. L., and Yeates, T. O. (2002) Arrangement of subunits and ordering of b-strands in an amyloid sheet. Nat. Struct. Biol. 9: 734-739.
Shin, Y. K., Levinthal, C., Levinthal, F., and Hubbell, W. L. (1993) Colicin E1 binding to membranes: time-resolved studies of spin-labeled mutants. Science 259: 960-963.
Singh, R. J., Feix, J. B., Mchaourab, H. S., Hogg, N., and Kalyanaraman, B. (1995) Spinlabeling study of the oxidative damage to low-density lipoprotein. Arch. Biochem. Biophys. 320: 155-161.
Smirnov, A. I., Smirnova, T. I., and Morse, P. D. (1995) Very high frequency electron paramagnetic resonance of 2,2,6,6-tetramethyl-1-piperidinyloxy in 1,2-dipalmitoyl-sn- glycero-3-phosphatidylcholine liposomes: partitioning and molecular dynamics. Biophys. J. 68: 2350-2360.
Steinhoff, H., Savitsky, A., Wegener, C., Pfeiffer, M., Plato, M., and Mobius, K. (2000) High-field EPR studies of the structure and conformational changes of site-directed spin labeled bacteriorhodopsin. Biochem. Biophys. Acta 1457: 253-262.
Steinhoff, H. J., Mollaaghababa, R., Altenbach, C., Hideg, K., Krebs, M., Khorana, H. G., and Hubbell, W. L. (1994) Time-resolved detection of structural changes during the photocycle of spin-labeled bacteriorhodopsin. Science 266: 105-107.
Stone, T. J., Buckman, T., Nordio, P. L., and McConnell, H. M. (1965) Spin-Labeled Biomolecules. Proc. Natl. Acad. Sci. USA 54: 1010-1017.
Sun, J., Voss, J., Hubbell, W. L., and Kaback, H. R. (1999) Proximity between periplasmic loops in the lactose permease of Escherichia coli as determined by site-directed spin labeling. Biochemistry 38: 3100-3105.
Thorgeirsson, T. E., Russell, C. J., King, D. S., and Shin, Y. K. (1996) Direct determination of the membrane affinities of individual amino acids. Biochemistry 35: 1803-1809.
Todd, A. P., Cong, J., Levinthal, F., Levinthal, C., and Hubbell, W. L. (1989) Site-directed mutagenesis of colicin E1 provides specific attachment sites for spin labels whose spectra are sensitive to local conformation. Proteins 6: 294-305.
Victor, K. and Cafiso, D. S. (1998) Structure and position of the N-terminal membranebinding domain of pp60src at the membrane interface. Biochemistry 37: 3402-3410.
Victor, K. G. and Cafiso, D. S. (2001) Location and dynamics of basic peptides at the membrane interface: electron paramagnetic resonance spectroscopy of tetramethyl- piperidine-N-oxyl-4-amino-4-carboxylic acid-labeled peptides. Biophys. J. 81: 2241-2250.
Vogelsang, M. S., Salwinski, L., and Hubbell, W. L. (2001) Membrane structure of 425-442 region of colicin E1 pore forming domain: a site-directed spin labeling study. Biophys. J. 80: 129a.
Voss, J., Hubbell, W. L., Hernandez-Borrell, J., and Kaback, H. R. (1997) Site-directed spinlabeling of transmembrane domain VII and the 4B1 antibody epitope in the lactose permease of Escherichia coli. Biochemistry 36: 15055-15061.
Voss, J., Hubbell, W. L., and Kaback, H. R. (1995a) Distance determination in proteins using designed metal ion binding sites and site-directed spin labeling: application to the lactose permease of Escherichia coli. Proc. Natl. Acad. Sci. USA 92: 12300-12303.
Voss, J., Salwinski, L., Kaback, H. R., and Hubbell, W. L. (1995b) A method for distance determination in proteins using a designed metal ion binding site and site-directed spin labeling: evaluation with T4 lysozyme. Proc. Natl. Acad. Sci. USA 92: 12295-12299.
308 CANDICE S. KLUG AND JIMMY B. FEIX
Voss, J., Wu, J., Hubbell, W. L., Jacques, V., Meares, C. F., and Kaback, H. R. (2001) Helix packing in the lactose permease of Escherichia coli: distances between site-directed nitroxides and a lanthanide. Biochemistry 40: 3184-3188.
Wang, Q., Voss, J., Hubbell, W. L., and Kaback, H. R. (1998) Proximity of helices VIII (Ala273) and IX (Met299) in the lactose permease of Escherichia coli. Biochemistry 37: 4910-4915.
Wertz, S. L., Savino, Y., and Cafiso, D. S. (1996) |
Solution and membrane bound structure of |
a peptide derived from the protein kinase |
C substrate domain of neuromodulin. |
Biochemistry 35: 11104-11112.
Wimley, W. C. and White, S. H. (1996) Experimentally determined hydrophobicity scale for proteins at membrane interfaces. [Review]. Nat. Struct. Biol. 3: 842-848.
Xiao, W. and Shin, Y. K. (2000) EPR Spectroscopic Ruler: the Method and its Applications in Biological Magnetic Resonance, Volume 19 (Berliner, L. J., Eaton, S. S., and Eaton, G. R., Eds.) pp 249-276, Kluwer Academic/Plenum Publishers, New York.
Yang, K., Farrens, D. L., Altenbach, C., Farahbakhsh, Z. T., Hubbell, W. L., and Khorana, H. G. (1996) Structure and function in rhodopsin. Cysteines 65 and 316 are in proximity in a rhodopsin mutant as indicated by disulfide formation and interactions between attached
spin labels. Biochemistry 35: 14040-14046. |
|
|
Yu, Y. G., Thorgeirsson, T. E., and Shin, Y. K. (1994) |
Topology of |
an amphiphilic |
mitochondrial signal sequence in the membrane-inserted |
state: a spin |
labeling study. |
Biochemistry 33: 14221 -14226. |
|
|
Zhao, M., Kalai, T., Hideg, K., Altenbach, C., Hubbell, W. L., and Kaback, H. R. (2000) Binding of spin-labeled galactosides to the lactose permease of Escherichia coli.
Biochemistry 39: 11381-11388.
Zhao, M., Zen, K. C., Hernandez-Borrell, J., Altenbach, C., Hubbell, W. L., and Kaback, H. R. (1999) Nitroxide scanning electron paramagnetic resonance of helices IV and V and the intervening loop in the lactose permease of Escherichia coli. Biochemistry 38: 1597015977.

310 |
DEREK MARSH ET AL. |
unremarkable and an essential development of the orientationally resolved method was the introduction of non-linear spectra detected in quadrature phase with the static field modulation (Hyde and Dalton, 1972). Such out-of- phase spectra have appreciable intensity only in the presence of saturation. The result is that the lineshapes of certain of these non-linear displays are exquisitely sensitive to rotational motion on the 
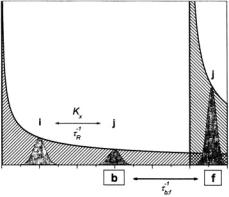
Figure 1. Schematic indication of saturation transfer processes (arrows) in axial powder spectra (heavy lines) for two ensembles of spins, b and f (hatched). Within ensemble b, saturation transfer takes place between orientationally selected component spin packets, i and j (grey), by internal exchange processes: either rotational diffusion 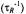 or Heisenberg spin exchange
or Heisenberg spin exchange 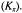 Two-site exchange leads to transfer of saturation between spin ensembles b and f, with characteristic rate constants
Two-site exchange leads to transfer of saturation between spin ensembles b and f, with characteristic rate constants 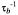 and
and 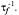
ST-EPR has come conventionally to be identified with studies of slow rotational motion. It is clear, indeed already to the originators, that this class of experiment can be used to study any type of slow molecular motion that gives rise to transfer of saturation particularly, for instance, two-site exchange (see Fig. 1). Further, the methods are applicable also to the quantitation of any magnetic interaction that alleviates saturation. Especially prominent among these are Heisenberg spin-exchange between nitroxides and interactions with paramagnetic relaxants such as transition metal complexes or molecular oxygen. The latter has been studied intensively by Hyde and coworkers, principally by saturation recovery EPR methods (Hyde and Subczynski, 1989). 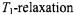 enhancement currently constitutes the most powerful EPR methodology in site-directed spin-labelling applications (Hubbell and Altenbach, 1994). The advantage of saturation-based methods is that they are sensitive to much weaker interactions than are the
enhancement currently constitutes the most powerful EPR methodology in site-directed spin-labelling applications (Hubbell and Altenbach, 1994). The advantage of saturation-based methods is that they are sensitive to much weaker interactions than are the
SATURATION TRANSFER SPECTROSCOPY |
311 |
conventional linewidths which are determined by the  rate rather than by the slower
rate rather than by the slower 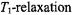 rate.
rate.
Here we aim to cover ST-EPR in its widest sense. Appropriate to the dedication of this volume, we begin with a description of the historical development of the subject. At its baldest this simply can be stated as: “nothing would have happened without Jim Hyde”. What follows is then devoted to a description of ST-spectroscopy up to its state-of-the-art application. Initial emphasis is placed on rotational diffusion measurements, but in-depth coverage is given also to the less conventional applications for studying exchange processes and paramagnetic relaxation enhancements. The advantage of integral methods is stressed throughout because they are generally insensitive to inhomogeneous broadening and the intensities are additive in multi-component systems.
2.HISTORICAL DEVELOPMENT
Saturation transfer spectroscopy has its origin in the observation by Hyde and collaborators (1970) that the CW saturation behaviour of the conventional low-temperature EPR spectra from flavin free radicals depended on temperature in a way compatible with slow molecular rotation. Following the classic analysis of adiabatic rapid-passage non-linear EPR spectra by Portis (1955) and Weger (1960), Hyde and Dalton (1972) explored the sensitivity to slow rotational diffusion of the first harmonic dispersion spectrum detected 90°-out-of-phase with respect to the field modulation. In the rigid limit, this  should have the pure (zeroth harmonic) absorption lineshape,
should have the pure (zeroth harmonic) absorption lineshape, 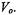 Rotation-dependent saturation transfer causes angularly selective decreases in intensity of the
Rotation-dependent saturation transfer causes angularly selective decreases in intensity of the 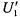 ST-EPR lineshape, relative to the absorption spectrum (Fajer and Marsh, 1983a). The predicted sensitivity of the
ST-EPR lineshape, relative to the absorption spectrum (Fajer and Marsh, 1983a). The predicted sensitivity of the  to slow motion was found for a small nitroxide spin label in supercooled glasses (Hyde and Dalton, 1972). Additionally, the ST-EPR spectra were found to depend on the fieldmodulation frequency,
to slow motion was found for a small nitroxide spin label in supercooled glasses (Hyde and Dalton, 1972). Additionally, the ST-EPR spectra were found to depend on the fieldmodulation frequency, 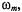 in a manner expected for rapid passage conditions. Essentially, the
in a manner expected for rapid passage conditions. Essentially, the 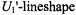 is determined by the product
is determined by the product 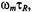 where
where 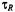 is the rotational correlation time. (Throughout this chapter, harmonics
is the rotational correlation time. (Throughout this chapter, harmonics  refer to a Fourier expansion of the EPR signal with respect to
refer to a Fourier expansion of the EPR signal with respect to 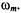 Experimentally, the various harmonic spectra are obtained by phasesensitive detection at frequencies
Experimentally, the various harmonic spectra are obtained by phasesensitive detection at frequencies 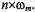 )
)
At the time, dispersion spectra were thought to be unsuitable for biological applications because of problems associated with sensitivity and klystron FM noise. A systematic search was therefore conducted by Hyde and Thomas (1973) to identify the out-of-phase ST-EPR display best suited
