
Biomedical EPR Part-B Methodology Instrumentation and Dynamics - Sandra R. Eaton
.pdf

384 |
ALBERT H. BETH AND ERIC J. HUSTEDT |
this chapter, |
can be utilized to predict the sensitivity of ST-EPR lineshapes |
to the correlation time for URD for a wide range of experimental parameters (microwave frequency and amplitude, modulation frequency and amplitude, harmonic and phase of the modulation, etc.) and for any selected geometry between the spin label reference frame and the URD axis. The calculated  spectra in Figure 3 show the dramatic changes in lineshapes that occur as a function of correlation time at 9.8 GHz when the labeling geometry is
spectra in Figure 3 show the dramatic changes in lineshapes that occur as a function of correlation time at 9.8 GHz when the labeling geometry is  45°, or 90°. These calculations, which show excellent agreement with previous calculations that were carried out using the eigenfunction expansion approach (Beth et al., 1983; Beth and Robinson, 1989), demonstrate that sensitivity to the correlation time for URD is minimal when the spin label z-axis is aligned with the diffusion axis and is maximum when the spin label z-axis is orthogonal to the diffusion axis. A major point to be made from these calculations is that the spectral shapes at, or near, major tilt angles of 0 or 90 deviate significantly from the lineshapes for isotopic rotational diffusion (i.e. there is no single correlation time for isorropic motion that will reproduce the spectra shown in Figure 3, left, and Figure 3, right, in the correlation time range from 1 to
45°, or 90°. These calculations, which show excellent agreement with previous calculations that were carried out using the eigenfunction expansion approach (Beth et al., 1983; Beth and Robinson, 1989), demonstrate that sensitivity to the correlation time for URD is minimal when the spin label z-axis is aligned with the diffusion axis and is maximum when the spin label z-axis is orthogonal to the diffusion axis. A major point to be made from these calculations is that the spectral shapes at, or near, major tilt angles of 0 or 90 deviate significantly from the lineshapes for isotopic rotational diffusion (i.e. there is no single correlation time for isorropic motion that will reproduce the spectra shown in Figure 3, left, and Figure 3, right, in the correlation time range from 1 to  ). At intermediate labeling geometries and at correlation times near 1 msec, however, the differences between URD and isotropic motion can be remarkably subtle (Hustedt and Beth, 1995).
). At intermediate labeling geometries and at correlation times near 1 msec, however, the differences between URD and isotropic motion can be remarkably subtle (Hustedt and Beth, 1995).
The calculations shown in Figure 3 were carried out using A- and g- tensor values and line widths that are appropriate for high resolution 
spin labels. However, the same sensitivities are predicted for conventional
spin labels (see Beth and Robinson, |
1989, for an extensive |
|
comparison of lineshapes from |
and |
spin labels). When |
carrying out spin labeling experiments on membrane proteins, it is generally not straightforward to predict a priori what the label geometry will be nor to alter it in those unfortunate cases where spectral sensitivity at X-band or lower microwave frequencies is minimized due to alignment, or near alignment, of the spin and diffusion tensors. When this situation is encountered, the higher microwave frequencies provide an increase in the minor element anisotropy due to the non-axial characteristics of the g-tensor and hence, increased sensitivity to motions that modulate the interconversion of the spin label x- and y-axes (see Beth and Robinson, 1989, for additional discussion of this point for ST-EPR studies at 3.0, 9.8, and 22 GHz).
Figure 4 demonstrates the high sensitivity of  signals at Q-band to the correlation time for URD and the spin label geometry. In particular, Figure 4, left, illustrates the dramatic increase in sensitivity to motional averaging of the nitroxide x- and y-axes by URD compared with the modest sensitivity at X-band (Figure 3, left). At the intermediate labeling geometry of 45° (Figure 4, middle), monotonic lineshape changes are seen throughout the
signals at Q-band to the correlation time for URD and the spin label geometry. In particular, Figure 4, left, illustrates the dramatic increase in sensitivity to motional averaging of the nitroxide x- and y-axes by URD compared with the modest sensitivity at X-band (Figure 3, left). At the intermediate labeling geometry of 45° (Figure 4, middle), monotonic lineshape changes are seen throughout the

SATURATION TRANSFER EPR |
385 |
spectrum as the correlation time increases in the range from  to 1 msec as reported previously for isotropic rotational diffusion (Johnson and Hyde, 1981). Alignment of the nitroxide x-axis with the URD axis (Figure 4, right) results in motional averaging of the y- and z-axes and large changes in lineshape in the high field end of the
to 1 msec as reported previously for isotropic rotational diffusion (Johnson and Hyde, 1981). Alignment of the nitroxide x-axis with the URD axis (Figure 4, right) results in motional averaging of the y- and z-axes and large changes in lineshape in the high field end of the 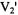 spectrum with almost no changes in the low field region. These results are entirely consistent with the predicted regions of spectral sensitivity in Figure 1, middle. These model calculations show that it is possible to get a qualitative sense of spin label geometry from simple inspection of the symmetry of the
spectrum with almost no changes in the low field region. These results are entirely consistent with the predicted regions of spectral sensitivity in Figure 1, middle. These model calculations show that it is possible to get a qualitative sense of spin label geometry from simple inspection of the symmetry of the  spectrum when using
spectrum when using  labels at Q-band. Even though the situation is more complex with
labels at Q-band. Even though the situation is more complex with  labels due to the severe overlap of turning points from the three nuclear manifolds in the central region of the spectrum, Q-band still provides improved sensitivity to the anisotropy of motion and spin label geometry relative to X-band (unpublished calculations). Early work by Johnson and coworkers (Johnson et al., 1982a; 1982b) demonstrated the advantages of Q- band ST-EPR to observe the effects of anisotropic motion.
labels due to the severe overlap of turning points from the three nuclear manifolds in the central region of the spectrum, Q-band still provides improved sensitivity to the anisotropy of motion and spin label geometry relative to X-band (unpublished calculations). Early work by Johnson and coworkers (Johnson et al., 1982a; 1982b) demonstrated the advantages of Q- band ST-EPR to observe the effects of anisotropic motion.
Figure 4. Calculated Q- band  ST-EPR spectra for a
ST-EPR spectra for a undergoing URD as
undergoing URD as
a function of |
(dottedline), |
(dashed dotted line), |
(solid line), |
|
and |
(dashed line) for |
(left panel), |
(middle panel), and |
(right |
panel); |
|
|
|
|
EPR spectrometers are now commercially available that provide excellent signal-to-noise ratio, stability, and reproducibility for carrying out ST-EPR experiments on aqueous samples at Q-band (e.g. Blackman et al., 2001). Given the significant improvements in sensitivity to details of anisotropic motion and spin label geometry, this microwave frequency should become a routine complement to X-band for detailed studies of rotational diffusion of any system undergoing anisotropic motion including the URD of intrinsic membrane proteins.

386 |
ALBERT H. BETH AND ERIC J. HUSTEDT |
W-band has recently emerged as an important complement to X- and Q- band for making EPR measurements on spin labels in aqueous solutions and in membrane systems (e.g. Smirnov et al., 1995; Hustedt et al., 1997; Gaffney and Marsh, 1998; Smirnov et al., 1998; Rohrer et al., 2001; Mangels et al., 2001). The higher magnetic field at W-band leads to complete resolution of the six turning points of a  label and the g- tensor anisotropy is larger than the A-tensor anisotropy (see Figure 1, right). The calculated
label and the g- tensor anisotropy is larger than the A-tensor anisotropy (see Figure 1, right). The calculated  spectra in Figure 5 show that like Q-band, W-band provides impressive sensitivity both to the correlation time for URD and the spin label geometry relative to the URD axis. To date, ST-EPR studies at W- band have not been reported in the literature. Therefore, there are no experimental data available to compare with these calculated lineshapes. It should be noted that calculations at W-band are more demanding than at the lower microwave frequencies both because of the need to calculate spectra over a wider range of magnetic fields and to couple over a finer orientational grid in order to achieve convergence. Efforts to compare spectra calculated with the transition rate matrix approach with those calculated using the eigenfunction expansion approach have been problematic due to the extreme number of eigenstates that have to be included in the calculations to approach convergence and the resulting very long computation times required.
spectra in Figure 5 show that like Q-band, W-band provides impressive sensitivity both to the correlation time for URD and the spin label geometry relative to the URD axis. To date, ST-EPR studies at W- band have not been reported in the literature. Therefore, there are no experimental data available to compare with these calculated lineshapes. It should be noted that calculations at W-band are more demanding than at the lower microwave frequencies both because of the need to calculate spectra over a wider range of magnetic fields and to couple over a finer orientational grid in order to achieve convergence. Efforts to compare spectra calculated with the transition rate matrix approach with those calculated using the eigenfunction expansion approach have been problematic due to the extreme number of eigenstates that have to be included in the calculations to approach convergence and the resulting very long computation times required.
Figure 5. Calculated W-band |
ST-EPR spectra for a |
undergoing URD as |
|||
a function of |
(dotted |
line), |
(dashed |
dotted line), |
(solid |
line), and |
(dashed line) for |
(left panel), |
(middle panel), |
and |
|
(right panel); |
|
|
|
|
|
The model calculations in Figure 5 indicate that ST-EPR studies at W- band could potentially provide a very useful avenue for investigation of membrane protein dynamics. However, it will be important to carefully choose systems that are amenable to investigation at this higher frequency due to sample volume limitations for aqueous samples using current

SATURATION TRANSFER EPR |
387 |
resonator designs. For those systems that can be concentrated into a small volume (e.g. 80 nL) and still maintain sample integrity, studies carried out at W-band are predicted to yield very detailed information on rotational dynamics throughout the very slow motional regime.
Figure 6. Calculated X-band (left panel), Q-band (middle panel), and W-band (right panel)
ST-EPR spectra for a |
|
undergoing URD with |
as a function of |
labeling geometry. |
(dotted line), |
(solid line), and |
(dashed line); |
The calculated spectra in Figure 6 further illustrate the range of ST-EPR lineshapes that are predicted at X-, Q-, and W-bands for three different orientations of the spin label reference frame relative to the URD axis. These spectra were all calculated for a URD model using a correlation time of 
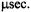 At X-band, the overall lineshapes are remarkably similar at tilt angles of 90 and 45° (Figure 6, left). It is not until the angle approaches 0° that the spectrum begins to deviate significantly from a fairly isotropic appearance. The net effect of this lack of sensitivity is that different models will fit the experimental data with rather similar values of
At X-band, the overall lineshapes are remarkably similar at tilt angles of 90 and 45° (Figure 6, left). It is not until the angle approaches 0° that the spectrum begins to deviate significantly from a fairly isotropic appearance. The net effect of this lack of sensitivity is that different models will fit the experimental data with rather similar values of  At Q-band, there is a steady change in overall lineshape from 0 to 90° as shown in Figure 6, middle. This sensitivity to orientation of the spin label is even more dramatic at W-band as shown in Figure 6, right. The spectra at 0 and at 90° at these higher frequencies are diagnostic of the label geometry and they are absolutely distinct from any isotropic motion lineshape. Since 45° is near the magic angle, the spectra at this angle appear similar to those for isotropic motion at a longer correlation time as predicted (Robinson and Dalton, 1980; Beth and Robinson, 1989). However, the lineshapes deviate from an isotropic appearance at the magic angle more quickly as the observer frequency is increased. These data suggest that it should be possible to recover much more accurate values for the correlation time for URD and the angle between the spin label and the URD axis at the higher microwave
At Q-band, there is a steady change in overall lineshape from 0 to 90° as shown in Figure 6, middle. This sensitivity to orientation of the spin label is even more dramatic at W-band as shown in Figure 6, right. The spectra at 0 and at 90° at these higher frequencies are diagnostic of the label geometry and they are absolutely distinct from any isotropic motion lineshape. Since 45° is near the magic angle, the spectra at this angle appear similar to those for isotropic motion at a longer correlation time as predicted (Robinson and Dalton, 1980; Beth and Robinson, 1989). However, the lineshapes deviate from an isotropic appearance at the magic angle more quickly as the observer frequency is increased. These data suggest that it should be possible to recover much more accurate values for the correlation time for URD and the angle between the spin label and the URD axis at the higher microwave

388 |
ALBERT H. BETH AND ERIC J. HUSTEDT |
frequencies using nonlinear least squares optimization between experiment and theory. Moreover, it should be possible to obtain better discrimination between different diffusion models at the higher frequencies except very near the magic angle.
Figure 7. Calculated X-band  ST-EPR spectra of a
ST-EPR spectra of a undergoing URD with
undergoing URD with
as a function of labeling geometry. |
(dotted line), |
(solid line), |
(dashed line); 
Most of the ST-EPR studies that have been carried out to-date have utilized normal isotope  labels and X-band. The improvements in spectral resolution and signal-to-noise ratio that are provided by the use of
labels and X-band. The improvements in spectral resolution and signal-to-noise ratio that are provided by the use of 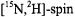 labels have been reviewed previously (Beth and Robinson, 1989) and will not be repeated in this chapter. However, it is instructive to make one direct comparison. The
labels have been reviewed previously (Beth and Robinson, 1989) and will not be repeated in this chapter. However, it is instructive to make one direct comparison. The  lineshapes in Figure 7 were calculated using tensor values and line widths that are appropriate for
lineshapes in Figure 7 were calculated using tensor values and line widths that are appropriate for  labels and the same motional model as in Figure 6. The major spectral change that is observed in going from 0° to 90° tilt of the spin label relative
labels and the same motional model as in Figure 6. The major spectral change that is observed in going from 0° to 90° tilt of the spin label relative

SATURATION TRANSFER EPR |
389 |
to the URD axis is apparent faster motion throughout the spectrum. Each of these calculated spectra has a remarkably isotropic appearance and each can be fit fairly convincingly using an isotropic rotational diffusion model and reasonable parameters for line width and a correlation time that is longer than the true correlation time for URD. This loss of discrimination between diffusion models represents a limitation for most studies of membrane protein dynamics. Going to higher microwave frequencies will improve the discrimination between different diffusion models. However, it will be more straightforward to analyze the data from  labels and to obtain more precise values for all parameters recovered from the nonlinear least squares fitting of experimental data. In most applications, the increase in signal-to-noise ratio, the increased resolution of spectral features, the better resolution of spectral turning points, and the decreased computation times will offset the modest expense of the isotopically substituted probes.
labels and to obtain more precise values for all parameters recovered from the nonlinear least squares fitting of experimental data. In most applications, the increase in signal-to-noise ratio, the increased resolution of spectral features, the better resolution of spectral turning points, and the decreased computation times will offset the modest expense of the isotopically substituted probes.
5.2Effects of Constrained URD on ST-EPR Lineshapes
Many intrinsic membrane proteins interact via their cytoplasmic domains with a variety of intracellular proteins including those that form the cytoskeleton. This type of interaction can restrict the global rotation of the transmembrane domain of an intrinsic membrane protein to an extent that will be determined by the flexibility of the linker region of the cytoplasmic domain (Nigg and Cherry, 1980; Matayoshi and Jovin, 1991; Blackman et al., 2001). When studies of rotational diffusion are carried out on membrane proteins in intact cell membranes, it is necessary to determine if there is a restriction in the amplitude of global URD in order to carry out a reliable analysis of experimental data.
Square-well restriction (Wahl, 1975) and harmonic-well restriction (Szabo, 1984) of URD has been considered for TOA. A restriction in the amplitude of URD results in an increase in  and a decrease in the apparent amplitudes of anisotropy decays with minimal effects on the rates of the decays (see Blackman et al., 2001). For most fluorophores and most label
and a decrease in the apparent amplitudes of anisotropy decays with minimal effects on the rates of the decays (see Blackman et al., 2001). For most fluorophores and most label
geometries, even weak restrictions (e.g. |
where |
is the full width of |
|
a square-well restriction) produce measurable |
changes |
in |
and there is |
excellent sensitivity to  in the range from 0 to approximately 100°.
in the range from 0 to approximately 100°.
Recent work has considered the effects of restricted amplitude URD on ST-EPR lineshapes (Hustedt and Beth, 2001). Figure 8 shows the
dependence of the |
signal on the full width |
of a square-well restriction |
at X- Q-, and W-band microwave frequencies for a URD model with |
||
and |
Three general conclusions |
are supported by these |
calculations. First, at all three microwave frequencies, there is excellent sensitivity to  between 0 and 30°, limited sensitivity between 30 and 90°,
between 0 and 30°, limited sensitivity between 30 and 90°,

390 |
ALBERT H. BETH AND ERIC J. HUSTEDT |
and almost no sensitivity between 90 and 360°. Sensitivity to  depends weakly on the spin label geometry relative to the URD axis. However, it is highly dependent on the correlation time for URD (Hustedt and Beth, 2001). At a correlation time of
depends weakly on the spin label geometry relative to the URD axis. However, it is highly dependent on the correlation time for URD (Hustedt and Beth, 2001). At a correlation time of  the
the  signal shows measurable changes out to at least 90°. At a correlation time of
signal shows measurable changes out to at least 90°. At a correlation time of  almost no changes in lineshape occur past 30°.
almost no changes in lineshape occur past 30°.
Figure 8. Calculated X-band (left panel), Q-band (middle panel), and W-band (right panel)
 ST-EPR spectra for a
ST-EPR spectra for a  undergoing restricted URD with
undergoing restricted URD with
and |
(dotted line), |
(dashed dotted line), |
(solid line), and |
 (dashed line).
(dashed line).
Second, Q- and W-band are only marginally more sensitive to large values of  than X-band. While this result might be unexpected, it should be noted that there has not been a systematic evaluation of the effects of various parameters, including modulation amplitude, on the sensitivity of these higher frequency measurements to
than X-band. While this result might be unexpected, it should be noted that there has not been a systematic evaluation of the effects of various parameters, including modulation amplitude, on the sensitivity of these higher frequency measurements to 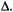 The sensitivity of ST-EPR to rotational dynamics is determined, in part, by the amplitude of the Zeeman modulation. For most biological samples, large Zeeman modulation amplitudes (typically 5 Gauss) are required to collect
The sensitivity of ST-EPR to rotational dynamics is determined, in part, by the amplitude of the Zeeman modulation. For most biological samples, large Zeeman modulation amplitudes (typically 5 Gauss) are required to collect  spectra with adequate signal-to-noise ratios. The use of higher modulation amplitudes leads to spectral broadening and to loss of resolution of spectral features without additional useful increases in the signal-to-noise ratio or increased sensitivity to rotational dynamics at X-band. However, the increased spectral width at the higher frequencies means that fewer orientations are sampled during a modulation
spectra with adequate signal-to-noise ratios. The use of higher modulation amplitudes leads to spectral broadening and to loss of resolution of spectral features without additional useful increases in the signal-to-noise ratio or increased sensitivity to rotational dynamics at X-band. However, the increased spectral width at the higher frequencies means that fewer orientations are sampled during a modulation

SATURATION TRANSFER EPR |
391 |
cycle. It may prove useful to evaluate the effects of modulation amplitude on the sensitivity of these higher frequency measurements to restricted amplitude motion in the future.
Third, at all three microwave frequencies, the |
signal |
changes |
|
dramatically for even small values of |
This is good |
news |
if one is |
interested in measuring a strong restriction on URD. However, it is troubling news with regards to the potential for unwanted contributions of local probe motion on  lineshapes. One limitation of ST-EPR is that the signals are dominated by the fastest motional processes that are present. The calculations in Figure 8 and those in previous work (Howard et al., 1993; Hustedt and Beth, 2001) indicate that even a 10° local mode of motion at a correlation time of
lineshapes. One limitation of ST-EPR is that the signals are dominated by the fastest motional processes that are present. The calculations in Figure 8 and those in previous work (Howard et al., 1993; Hustedt and Beth, 2001) indicate that even a 10° local mode of motion at a correlation time of  or less, will give the appearance of significantly faster overall motion thereby compromising determination of the true global rotational diffusion of the system. Very little work has been directed at designing spin labels that are rigidly coupled to target proteins, particularly since the advent of site directed spin labeling approaches. This is an area that should be emphasized as methods for quantitative analysis of ST-EPR data evolve.
or less, will give the appearance of significantly faster overall motion thereby compromising determination of the true global rotational diffusion of the system. Very little work has been directed at designing spin labels that are rigidly coupled to target proteins, particularly since the advent of site directed spin labeling approaches. This is an area that should be emphasized as methods for quantitative analysis of ST-EPR data evolve.
5.3Opportunities for Further Advances in ST-EPR
ST-EPR lineshapes are dependent on the amplitudes of the microwave observer field and the Zeeman modulation field at the sample. Also, since they are detected 90° out-of-phase with respect to the field modulation and the in-phase signals are much larger in amplitude, they are very dependent on the phase of the modulation at the sample. Most X-band ST-EPR studies to-date have been carried out in conventional  or
or  cavities with sample volumes ranging from
cavities with sample volumes ranging from  (in capillaries) to
(in capillaries) to  (in agueous flat cells). The sample geometry normally employed gives rise to a distribution of microwave and modulation fields and to phase shifts over the active dimensions of the sample (see Fajer and Marsh, 1982; Hemminga and de Jager, 1989). Figure 9 shows the experimentally measured values for each of these parameters over a 3.2 cm long aqueous sample positioned vertically in a commercial Bruker
(in agueous flat cells). The sample geometry normally employed gives rise to a distribution of microwave and modulation fields and to phase shifts over the active dimensions of the sample (see Fajer and Marsh, 1982; Hemminga and de Jager, 1989). Figure 9 shows the experimentally measured values for each of these parameters over a 3.2 cm long aqueous sample positioned vertically in a commercial Bruker  resonator. Also shown are the calculated
resonator. Also shown are the calculated  signals from a point sample positioned at the center of the cavity (defined operationally as the position where
signals from a point sample positioned at the center of the cavity (defined operationally as the position where  and
and  are maximum) and the calculated composite signal that explicitly accounts for each of these distributions. These calculations show that there can be discernable distortions of the experimental lineshape even with a moderate sample size. For samples that are amenable, such as membrane proteins reconstituted in liposomes at high effective concentrations, loop-gap resonators may provide better characteristics due to the smaller sample size. Though extensive
are maximum) and the calculated composite signal that explicitly accounts for each of these distributions. These calculations show that there can be discernable distortions of the experimental lineshape even with a moderate sample size. For samples that are amenable, such as membrane proteins reconstituted in liposomes at high effective concentrations, loop-gap resonators may provide better characteristics due to the smaller sample size. Though extensive

392 |
ALBERT H. BETH AND ERIC J. HUSTEDT |
studies have not been carried out in commercial  resonators at Q-band, preliminary work has indicated that the distribution of modulation amplitudes at this higher frequency is appreciable, perhaps worse than at X- band, even with the small sample volumes normally employed.
resonators at Q-band, preliminary work has indicated that the distribution of modulation amplitudes at this higher frequency is appreciable, perhaps worse than at X- band, even with the small sample volumes normally employed.
Figure 9. Left: Variation of  (plus signs),
(plus signs),  (solid squares), and modulation phase (open circles) as a function of sample position in a
(solid squares), and modulation phase (open circles) as a function of sample position in a  cavity. These values were measured using a point sample of PADS in a
cavity. These values were measured using a point sample of PADS in a  capillary. Right: Calculated
capillary. Right: Calculated  ST-EPR spectra for a point sample at the center position (solid line) and a composite signal calculated for a 3.2 cm long sample (dotted line);
ST-EPR spectra for a point sample at the center position (solid line) and a composite signal calculated for a 3.2 cm long sample (dotted line); 
In theory, it would be straightforward to include each of these distributions in the nonlinear least squares fitting of experimental data. However, this added complexity results in a very demanding computational problem given the number of additional lineshapes that have to be calculated at each iteration. Recent work by Hyde and coworkers (Mett et al., 2001; Anderson et al., 2002) has shown that alternative cavity structures can be designed that provide a homogeneous  field over an extended sample volume. These structures may prove extremely useful for future ST-EPR applications and for other EPR investigations that require nonlinear
field over an extended sample volume. These structures may prove extremely useful for future ST-EPR applications and for other EPR investigations that require nonlinear  fields.
fields.

 Thus, the global analysis of data obtained at different
Thus, the global analysis of data obtained at different  should aid in defining the relationship between the diffusion axis and the spin label for anisotropic rotational diffusion models such as URD. This is an important avenue for future applications of ST-EPR.
should aid in defining the relationship between the diffusion axis and the spin label for anisotropic rotational diffusion models such as URD. This is an important avenue for future applications of ST-EPR. Label Geometry, and Microwave Frequency.
Label Geometry, and Microwave Frequency.