
Biomedical EPR Part-B Methodology Instrumentation and Dynamics - Sandra R. Eaton
.pdf282 CANDICE S. KLUG AND JIMMY B. FEIX
The effects of different neighboring side chains on the motion of the spin label in  was studied in the
was studied in the  CRBP (Lietzow and Hubbell, 1998; Hubbell et al., 1998). It was concluded that nearest-neighbor solventaccessible side chain interactions strongly influenced spin label motion. This was determined by mutating neighboring side chains and observing the spectral effects on a given spin labeled site. These findings were in contrast to those found in an
CRBP (Lietzow and Hubbell, 1998; Hubbell et al., 1998). It was concluded that nearest-neighbor solventaccessible side chain interactions strongly influenced spin label motion. This was determined by mutating neighboring side chains and observing the spectral effects on a given spin labeled site. These findings were in contrast to those found in an  model system, T4L, where side chain dynamics of solvent-exposed helical sites are less dependent on nearest neighbors (Mchaourab et al., 1996). Solvent-exposed sites in
model system, T4L, where side chain dynamics of solvent-exposed helical sites are less dependent on nearest neighbors (Mchaourab et al., 1996). Solvent-exposed sites in 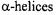 appear to be largely dependent on backbone mobility and tertiary, rather than secondary, interactions. The use of spin labels other than MTSL, including the more flexible saturated derivative of MTSL, may also allow better spectral contrast between surface site spin labels and buried labels (Mchaourab et al., 1999).
appear to be largely dependent on backbone mobility and tertiary, rather than secondary, interactions. The use of spin labels other than MTSL, including the more flexible saturated derivative of MTSL, may also allow better spectral contrast between surface site spin labels and buried labels (Mchaourab et al., 1999).
Changes in mobility and the resultant alteration in signal amplitude at a given spectral position are also the basis for the majority of SDSL studies on time-resolved conformational changes. Often this involves simply “sitting” on a sensitive spectral position such as the maxima of a stronglyor weaklyimmobilized peak and observing changes in intensity with time. For example, the kinetics of rhodopsin photoactivation (Farahbakhsh et al., 1993) and the bacteriorhodopsin photocycle (Rink et al., 1997; Mollaaghababa et al., 2000) were followed by optical methods and compared to the kinetics of motional changes at a given spin-labeled site. Agreement not only shows that the relevant biological process is being observed, but also indicates whether the labeled site in question is undergoing a change in local structural environment and to what degree the structural changes and biological processes are coupled. Time-resolved studies have also been used to monitor rigid-body movements associated with the bacteriorhodopsin photocycle (Steinhoff et al., 1994) and membrane insertion of colicin E1 (Shin et al., 1993).
The SDSL approach is also being used in RNA research. Recently, nitroxide derivatives have been introduced at specific backbone locations in an RNA hairpin through a deoxyribo-phosphorothiolate linkage to detect, for the first time, GAAA tetraloop/receptor complex formation (Qin et al., 2001). Here, a method was presented to specifically label internal locations of the RNA backbone independent of the sequence and it was found that the modification and spin labeling of the tetraloop hairpin did not significantly perturb the secondary structure of RNA. The free energy of complex formation and a 
 were determined from EPR motional parameters and laid the foundation for further work on the quantification of weak interactions in nucleic acids and nucleic acid/protein complexes.
were determined from EPR motional parameters and laid the foundation for further work on the quantification of weak interactions in nucleic acids and nucleic acid/protein complexes.
SDSL: A SURVEY OF BIOLOGICAL APPLICATIONS |
283 |
In addition, other methods for spin labeling RNA have been published. For example, a 4-thiol was substituted in an unpaired uridine and spin labeled for the detection of long-range RNA/protein interactions by NMR (Ramos and Varani, 1998), an amino-specific spin label was used to label the 2’-ammo-modified position of base-paired nucleotides to investigate the trans-activation responsive region of HIV RNA by EPR (Edwards et al., 2001), and Shin and coworkers have used 5’ displacement spin labeling by incorporating a guanosine monophosphorothioate at the 5’ end of Rev response element, allowing labeling of the 5’ end of an RNA molecule (Macosko et al., 1999). Spin labels have also been used in the study of DNA by conjugation to either the sugar-phosphate backbone or a nucleoside base (e.g. (Miller et al., 1995)).
3.2Protein backbone flexibility
In addition to studying the motion of the protein itself and the motion of the spin label side chain attached to specific sites within a protein, fluctuations in the backbone motion can also be detected by analysis of the spectral dynamics (Columbus and Hubbell, 2002) or with the use of novel modifications to the commonly-used MTSL. Additions to the MTSL nitroxide 4’ position (Figure 4) have very recently been shown to give valuable information on the origin of spectral motion (Columbus et al., 2001). The addition of a 4-methyl group restricts motion about the  and
and  bonds of MTSL (Mchaourab et al., 1996; Columbus et al., 2001), while the addition of a 4-phenyl group restricts motion about the remaining bonds, leaving spectral motion sensitive only to backbone fluctuations. This was demonstrated for the first time in the workhorse, T4L, at an external helix site and very nicely illustrated the gradual hindering of spin label motion and the backbone rigidity of the helix (Columbus et al., 2001). A 4-bromo derivative of MTSL was also used to restrict side chain motion and thus improve distance measurements in double-label T4L experiments (Altenbach et al., 2001c). These are unique methods of gathering information about the flexibility of protein backbones in very localized regions and could reveal important information on the function of various secondary structural folds. Although this is a recent advance in SDSL, it likely will become common practice in the near future due to its ability to systematically restrict motion of the spin label side chain and yield direct backbone dynamics.
bonds of MTSL (Mchaourab et al., 1996; Columbus et al., 2001), while the addition of a 4-phenyl group restricts motion about the remaining bonds, leaving spectral motion sensitive only to backbone fluctuations. This was demonstrated for the first time in the workhorse, T4L, at an external helix site and very nicely illustrated the gradual hindering of spin label motion and the backbone rigidity of the helix (Columbus et al., 2001). A 4-bromo derivative of MTSL was also used to restrict side chain motion and thus improve distance measurements in double-label T4L experiments (Altenbach et al., 2001c). These are unique methods of gathering information about the flexibility of protein backbones in very localized regions and could reveal important information on the function of various secondary structural folds. Although this is a recent advance in SDSL, it likely will become common practice in the near future due to its ability to systematically restrict motion of the spin label side chain and yield direct backbone dynamics.
284 |
CANDICE S. KLUG AND JIMMY B. FEIX |
Figure 4. MTSL 4’ modifications. MTSL (–H), with 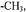 and
and  modifications. Arrows indicate the
modifications. Arrows indicate the  and
and bond rotations. When the labels are covalently attached to a cysteine residue,
bond rotations. When the labels are covalently attached to a cysteine residue, and
and 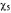 arethe
arethe  and
and 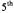 bonds from the
bonds from the 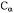 of the peptide backbone (see Figure 1).
of the peptide backbone (see Figure 1).
3.3Protein-substrate and protein-protein interactions
The myristoylated alanine-rich protein kinase C substrate (MARCKS) is thought to sequester phosphoinositides within the lipid bilayer. SDSL studies of spin labeled MARCKS peptides indicated that the peptide did indeed sequester phosphatidylinositol 4,5-bisphosphate 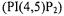 in the membrane, that this mechanism was driven by electrostatic interactions, and that MARCKS did not significantly alter its structure upon binding to
in the membrane, that this mechanism was driven by electrostatic interactions, and that MARCKS did not significantly alter its structure upon binding to  (Rauch et al., 2002). Also interesting in this study was the use of spin labeled
(Rauch et al., 2002). Also interesting in this study was the use of spin labeled 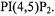
 was synthesized, characterized, and used to directly study the sequestration of
was synthesized, characterized, and used to directly study the sequestration of  in the membrane, which confirmed the hypothesis that MARCKS interacts with multiple
in the membrane, which confirmed the hypothesis that MARCKS interacts with multiple  molecules. In both cases, the motional changes in the spectra were followed by a function of the amplitude of the center spectral lineheight and bilayer depth measurements of the MARCKS peptide.
molecules. In both cases, the motional changes in the spectra were followed by a function of the amplitude of the center spectral lineheight and bilayer depth measurements of the MARCKS peptide.
Conformational changes have been seen in the SNARE (soluble NSF acceptor protein receptor) complexes, which appear to be able to switch between helical and random structures either in part or as a whole in a homoor heterooligomeric complex–dependent manner (Margittai et al., 2001). SNARE complex assembly is necessary for membrane fusion and neurotransmitter release in neurons. SDSL nitroxide scanning studies showed that the membrane/water interfacial domain inserts into the bilayer as the membrane coupling step in membrane fusion (Kweon et al., 2002). Another study on the assembly of the SNARE complex after membrane insertion revealed a possible fusion mechanism first involving the insertion of the membrane domains followed by the assembly of the complex to pull the membranes together for fusion (Kim et al., 2002).
Coupling through trimeric G-proteins is one of the most important signal transduction mechanisms in biology. A 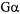 protein lacking reactive cysteine
protein lacking reactive cysteine

SDSL: A SURVEY OF BIOLOGICAL APPLICATIONS |
285 |
residues was engineered and a series of single-cysteine mutations introduced in the functionally-important N-terminus, a region of the protein that is absent in the relevant crystal structures. SDSL showed that this region of the
isolated protein |
is disordered, but adopts an |
structure upon |
interaction with |
(Medkova el al., 2002). |
|
BtuB is an outer membrane protein similar to FepA that is responsible for the uptake of vitamin  and requires interaction with TonB in order to translocate the bound ligand. SDSL studies were carried out on the TonB box, the N-terminal segment of BtuB that is thought to interact with TonB once ligand is bound to the extracellular loops of the receptor, both before and after addition of cyanocobalamin ligand (Merianos et al., 2000). It was found that this region is a structured helix located within the barrel of the receptor prior to ligand binding, but converts to an extended, disordered segment that likely extends into the periplasm after ligand is bound. In transport-defective mutants of BtuB this region of the protein was unstructured even in the resting state (Coggshall et al., 2001) and showed no evidence of a conformational change upon ligand binding. Both spectral motion and accessibility measurements were utilized in identifying the nature of the ligand-induced conformational change. In addition, experiments were carried out in intact outer membrane preparations rather than purifying and reconstituting the protein. Background labeling was found to be less than 10% of the total signal and the native lipid environment of the receptor was maintained.
and requires interaction with TonB in order to translocate the bound ligand. SDSL studies were carried out on the TonB box, the N-terminal segment of BtuB that is thought to interact with TonB once ligand is bound to the extracellular loops of the receptor, both before and after addition of cyanocobalamin ligand (Merianos et al., 2000). It was found that this region is a structured helix located within the barrel of the receptor prior to ligand binding, but converts to an extended, disordered segment that likely extends into the periplasm after ligand is bound. In transport-defective mutants of BtuB this region of the protein was unstructured even in the resting state (Coggshall et al., 2001) and showed no evidence of a conformational change upon ligand binding. Both spectral motion and accessibility measurements were utilized in identifying the nature of the ligand-induced conformational change. In addition, experiments were carried out in intact outer membrane preparations rather than purifying and reconstituting the protein. Background labeling was found to be less than 10% of the total signal and the native lipid environment of the receptor was maintained.
Conformational changes near the ferric enterobactin binding loops of FepA showed a dramatic conformational change upon the binding of ligand (Klug et al., 1998) along with a corresponding change in accessibility measurements. In addition, FepA is the receptor for the toxins colicins B and D, for which the mechanism of binding is unknown. Changes in the spectrum were also seen upon the addition of colicin B, but were not as dramatic, indicating that this site is involved in structural rearrangements when both ligands bind, strengthening the theory that distinct binding mechanisms exist for each ligand (unpublished results, C. Klug and J. Feix).
3.4Protein folding and denaturation
The formation or loss of motional constraints that arise from local tertiary structure provides a sensitive means by which to monitor protein folding or denaturation, respectively. For many systems, chemical denaturation with detergents or compounds that disrupt protein-solvent interactions (e.g., urea or guanidine hydrochloride) is a reversible process, so that determination of the equilibrium between folded and unfolded species as a function of denaturant concentration gives direct insight into the thermodynamic
286 CANDICE S. KLUG AND JIMMY B. FEIX
parameters that characterize protein stability. Since the motional properties of an attached spin label will in general be different for the folded and unfolded states, SDSL provides a method for determining these parameters at various sites throughout the protein structure.
Initial studies on FepA indicated that denaturation of a site near the extracellular ligand binding site was well-described by a two-state equilibrium between folded and unfolded species, thus providing a measure of the Gibbs free energy of unfolding (Klug et al., 1995). MTSL bound to this particular site (E280C) was strongly immobilized in the native state, but became freely mobile upon denaturation with both urea and guanidine hydrochloride. Additional studies on the denaturation of FepA have shown this to be generally true for  residues facing the interior, globular domain that fills the channel (Klug and Feix, 1998). It should be noted that free energies of denaturation determined by SDSL are specific for the site examined, i.e. they reflect a local loss of tertiary structure around the nitroxide side chain rather than global unfolding of the protein. The latter can be determined by more non-specific methods such as circular dichroism (CD) or calorimetry. This is also observed with other site-specific methods, for example when hydrogen-deuterium exchange is monitored by NMR or mass spectroscopy, each residue will display a specific free energy of denaturation and these may vary widely within a given protein.
residues facing the interior, globular domain that fills the channel (Klug and Feix, 1998). It should be noted that free energies of denaturation determined by SDSL are specific for the site examined, i.e. they reflect a local loss of tertiary structure around the nitroxide side chain rather than global unfolding of the protein. The latter can be determined by more non-specific methods such as circular dichroism (CD) or calorimetry. This is also observed with other site-specific methods, for example when hydrogen-deuterium exchange is monitored by NMR or mass spectroscopy, each residue will display a specific free energy of denaturation and these may vary widely within a given protein.
The specificity of denaturation measurements by SDSL for a given labeling site provides the opportunity to compare the stability of different structural elements or domains within a given protein. Similar studies using more global methods such as CD are typically done by expressing each domain independently and measuring their stability in isolation. This can give erroneous results if there are important contacts between domains. In addition, SDSL can be used to examine the effects of mutations on protein stability by maintaining the spin label at a given location and measuring the effects of mutations at other sites of interest. Clearly, there are numerous advantages to the SDSL approach in these types of studies.
Scholes and co-workers have employed time-resolved SDSL to examine the folding of cytochrome c (Grigoryants et al., 2000; DeWeerd et al., 2001). Multiple rate constants were observed, demonstrating the complexity of this biological process even for relatively small proteins. Instrumentation was developed and refined allowing observation of kinetic components on the 0.1 msec time scale. This methodology has tremendous potential for examining early events in protein folding and denaturation.

SDSL: A SURVEY OF BIOLOGICAL APPLICATIONS |
287 |
3.5Peptide-Membrane Interactions
A change in the mobility of a peptide-bound spin label upon membrane binding forms the basis for a sensitive method to measure peptide-membrane binding affinity. In the aqueous phase, peptides and small proteins are generally unstructured and/or rapidly tumbling, leading to a nearly isotropic spectrum with rotational motion on the subnanosecond time scale. Peptide binding to a liposome or cell surface restricts mobility, producing a twocomponent spectrum that is a superposition of signals arising from free and bound peptide. Because the high-field 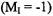 line of the bound peptide is relatively broad (Figure 5), it contributes little to the peak-peak amplitude of the high-field line, so that simple measurement of this amplitude allows rapid determination of the fractions of free and bound peptide. This leads to ready determination of membrane binding affinities and partition coefficients that are essential parameters for understanding the physical basis of peptide-membrane interactions.
line of the bound peptide is relatively broad (Figure 5), it contributes little to the peak-peak amplitude of the high-field line, so that simple measurement of this amplitude allows rapid determination of the fractions of free and bound peptide. This leads to ready determination of membrane binding affinities and partition coefficients that are essential parameters for understanding the physical basis of peptide-membrane interactions.
Figure 5. EPR spectra of peptides. Spin labeled peptides free in solution (top) and bound to membranes(bottom).
An excellent example of this methodology is a recent study of fiveand six-residue model peptides containing lysine and phenylalanine that were analyzed to determine the relative contributions of hydrophobic and electrostatic interactions to membrane association (Victor and Cafiso, 2001). These peptides bind membranes with partition coefficients that vary from < 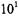 to >
to >  depending on peptide composition and the mol fraction of negatively charged lipid. In contrast, the hydrophobic fungal peptide
depending on peptide composition and the mol fraction of negatively charged lipid. In contrast, the hydrophobic fungal peptide
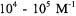
288 CANDICE S. KLUG AND JIMMY B. FEIX
antibiotic alamethicin binds even to neutral bilayers with partition
coefficients on the order of |
(Archer et al., 1991; Lewis and |
Cafiso, 1999). A spin-labeled |
analog of the 33-residue insect peptide |
antibiotic cecropin binds to membranes containing 30 mol % PG with a partition coefficient of  (Mchaourab et al., 1994), and use of this methodology has allowed us to rapidly evaluate binding affinities for a number of modified cecropin analogs (J. Feix, unpublished data).
(Mchaourab et al., 1994), and use of this methodology has allowed us to rapidly evaluate binding affinities for a number of modified cecropin analogs (J. Feix, unpublished data).
The ability to design membrane-binding peptides and the development of insights into the forces governing insertion of integral membrane proteins requires an understanding of the relative affinities of the individual amino acids for the hydrophobic phase of the bilayer. A number of relative thermodynamic scales have been developed, such as those of Wimley and White (Wimley and White, 1996) and Li and Deber (Li and Deber, 1994). Shin and co-workers have used SDSL to develop a similar scale for hydrophobic propensity (Thorgeirsson et al., 1996). They employed a hostguest system based on the membrane binding presequence of yeast cytochrome c oxidase, with each peptide containing a spin-labeled site and a second site for the guest amino acid, to determine the free energy of transfer  from aqueous solution to the membrane. Changes in
from aqueous solution to the membrane. Changes in 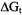 relative to glycine were in good agreement with those determined from octanol:water partition coefficients of the isolated N-acetyl amino acid amides. A study on the temperature-dependence of membrane partitioning for four of these peptides indicated that membrane binding was largely entropy-driven (Russell et al., 1996). A subsequent study that employed a designed
relative to glycine were in good agreement with those determined from octanol:water partition coefficients of the isolated N-acetyl amino acid amides. A study on the temperature-dependence of membrane partitioning for four of these peptides indicated that membrane binding was largely entropy-driven (Russell et al., 1996). A subsequent study that employed a designed 
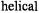 peptide as the host suggested that hydrophobic propensity measured in this manner was not dependent on the site at which the guest peptide was introduced (Russell et al., 1999). Thus, SDSL allowed analysis of this fundamental physical property in a context much more biologically relevant than simple partitioning between aqueous solution and an organic solvent.
peptide as the host suggested that hydrophobic propensity measured in this manner was not dependent on the site at which the guest peptide was introduced (Russell et al., 1999). Thus, SDSL allowed analysis of this fundamental physical property in a context much more biologically relevant than simple partitioning between aqueous solution and an organic solvent.
3.6Additional approaches
In addition to the standard approach of site-specific cysteine incorporation followed by specific labeling of the -SH group with MTSL, peptides prepared by solid-phase peptide synthesis (SPPS) can incorporate the nitroxide amino acid TOAC (2,2,6,6-tetramethylpiperidine-1-oxyl-4-amino- 4-carboxylic acid; Figure 6) (e.g. Marchetto et al., 1993, Hanson et al., 1996; McNulty et al., 2000; Victor and Cafiso, 2001), reviewed by (McNulty and Millhauser, 2000)). The TOAC label is tightly coupled to the peptide backbone, and the nitroxide moiety lies only ~ 2.4Å from  TOAC incorporation appears to stabilize
TOAC incorporation appears to stabilize  and
and 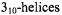 (Hanson et al., 1996). Recently, a method was described for coupling a carboxylate spin
(Hanson et al., 1996). Recently, a method was described for coupling a carboxylate spin

SDSL: A SURVEY OF BIOLOGICAL APPLICATIONS |
289 |
label to a diaminopropionic acid (Dap) residue during SPPS (McNulty et al., 2002). The Dap-spin label side chain (Figure 7) is expected to be less perturbing than the TOAC label, especially at non-helical sites. Amino group-specific succinimide spin labels can also be used to label small peptides and proteins, binding to lysine residues or the N-terminus (Altenbach et al., 1989b; Archer et al., 1991), however most proteins have far too many reactive amino groups to make this approach very useful.
Figure 6. TOAC. Spin label |
Figure 7. Dap-Spin Label. |
used in peptide synthesis. |
|
Additional spin labels other than MTSL and its analogs can also be used to label the cysteine sulfhydryl group. Traditionally, maleimide (e.g. (Singh et al., 1995; Hustedt and Beth, 1996; Blackman et al., 2001) and iodoacetamide (e.g. Panse et al., 2001; Kersten et al., 2000) labels have been used with good success. Although their absolute chemical specificity for cysteine is not as high as the methanethiosulfonates, they do have the advantage of being chemically stable in the presence of reducing agents. It is also likely that there will continue to be numerous modifications and adaptations of MTSL (some of which are discussed above), such as the addition of charged or polar groups that more accurately mimic the native amino acid side chain being replaced.
It is becoming apparent that a multifrequency approach, including the use of Q- and W-band as well as even higher frequencies, will provide a more complete characterization of a given labeling site (Borbat et al., 2001). At frequencies of 94GHz and higher, the spin label spectrum is more sensitive to the faster motional dynamics range and shows better resolution of the g and A tensors for anisotropic motion. These benefits have already been useful for the study of membranes and membrane proteins (Mangels et al., 2001; Smirnov et al., 1995; Steinhoff et al., 2000; Barnes et al., 1999; McNulty et al., 2000; Bennati et al., 1999; Borbat et al., 2002). In addition, dipolar couplings between sets of two spin labels are more accurately
290 |
CANDICE S. KLUG AND JIMMY B. FEIX |
analyzed at higher frequencies (Hustedt et al., 1997; Hustedt and Beth, 1999; McNulty et al., 2000).
Also, pulsed and double quantum EPR methods that provide more direct insights into relaxation properties are likely to play an increasingly important role in future SDSL studies (Borbat et al., 2001; Eaton et al., 2000). These approaches are particularly powerful for making nitroxide-metal and nitroxide-nitroxide distance measurements (discussed below).
4.DISTANCE MEASUREMENTS
Distances between two nitroxides, or a nitroxide and a metal ion, can provide information on both protein structure and functional dynamics. SDSL EPR can give information on distances between spin labels within the range of about 8-25Å using CW methods, and of 50Å or greater using pulse methods (extensively reviewed in Volume 19 of this series). Various methodologies have been developed over the years to study the interaction of spin labels in biological systems. For example, methods exist for data acquisition in frozen solution or at room temperature, and various programs exist to analyze and quantitate the spectra that result from spin–spin interactions. The ability to monitor conformational changes within a protein due to structural rearrangement is a unique benefit of this technique.
4.1Acquisition and Analysis Methodologies
A growing application of SDSL is the measurement and analysis of magnetic dipolar interactions between two spin labels to determine interspin distance. Following initial mapping of 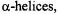 loops, and
loops, and  distance measurements can provide insight into how these secondary structural elements pack in the tertiary structure of the protein, and the observation of alterations in distance between sites upon ligand binding or protein-protein interaction is a powerful approach to characterizing conformational changes. Determination of spin-spin distances between different monomeric components can provide insights into the quaternary organization of a macromolecular complex. Some of the more commonly used approaches for measuring interspin distance are discussed below in representative applications.
distance measurements can provide insight into how these secondary structural elements pack in the tertiary structure of the protein, and the observation of alterations in distance between sites upon ligand binding or protein-protein interaction is a powerful approach to characterizing conformational changes. Determination of spin-spin distances between different monomeric components can provide insights into the quaternary organization of a macromolecular complex. Some of the more commonly used approaches for measuring interspin distance are discussed below in representative applications.
Rabenstein and Shin introduced a method for determination of interspin distance based on Fourier transform deconvolution of dipolar-coupled spectra (Rabenstein and Shin, 1995; Xiao and Shin, 2000). The EPR spectra of interacting spins were treated as a convolution of non-interacting powder pattern spectra with a dipolar broadening function as described by Pake

SDSL: A SURVEY OF BIOLOGICAL APPLICATIONS |
291 |
|
(Pake, 1948). This approach was validated |
using a |
series of lysine- |
containing polyalanine peptides with the general sequence |
||
that folds into a well-defined |
Pairs |
of cysteines were |
substituted for alanine residues at various distances apart, labeled with MTSL, and their spectra obtained in frozen solution. Excellent agreement between the measured distances and those based on a molecular model was obtained for sites spaced between 7 and 25Å (Rabenstein and Shin, 1995). Modeling necessarily must take into consideration the size of the nitroxide side chain, and this study found the best fit for an arm length (from the 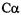 carbon to the nitroxide) of 6.7Å, in good agreement with theoretical expectation. One drawback of this method is the requirement to have rigidlimit spectra, which will usually require freezing of the sample. A strength, however, is the ability to accurately determine distances even in the presence of singly-labeled species. This is of particular importance since it is often difficult to attain stoichiometric labeling.
carbon to the nitroxide) of 6.7Å, in good agreement with theoretical expectation. One drawback of this method is the requirement to have rigidlimit spectra, which will usually require freezing of the sample. A strength, however, is the ability to accurately determine distances even in the presence of singly-labeled species. This is of particular importance since it is often difficult to attain stoichiometric labeling.
The Rabenstein and Shin approach has been utilized in a large number of double-labeling applications including studies of HIV gp41 peptides (Rabenstein and Shin, 1996), the KcsA potassium channel (Perozo et al., 1998; Perozo et al., 1999; Liu et al., 2001; Gross et al., 1999), the mechanosensitive MscL channel (Perozo et al., 2002b), the neuronal SNARE complex (Kim et al., 2002), and the inhibitory component of cardiac muscle troponin (Brown et al., 2002).
Hustedt and Beth have developed rigorous simulation methodologies that provide the most precise assessment of distance and relative orientation between two nitroxides currently available (Hustedt et al., 1997; Hustedt and Beth, 2000), Their approach determines all of the independent variables describing the spatial relationship of the two nitroxides, including the interspin distance and five independent angles. The accuracy of the fit is significantly enhanced by obtaining spectra at multiple frequencies, and this study utilized data at X-, Q-, and W-band. Resolution was also enhanced by using perdeuterated spin labels to reduce inhomogeneous broadening. The distance between two spin-labeled  analogs bound to glyceraldehyde- 3-phosphate dehydrogenase was measured as 12.85Å with 99% confidence levels on the order of 0.1 – 0.2Å. It should be noted that the resolution attained in this approach depends on having two labels at a fixed distance and in a fixed relative orientation. These conditions are not often achieved in SDSL, except in the case of buried, strongly-immobilized sites. Further development to accommodate the distribution of distances and angles normally found with spin labeled cysteine residues is in progress (Hustedt and Beth, 2000).
analogs bound to glyceraldehyde- 3-phosphate dehydrogenase was measured as 12.85Å with 99% confidence levels on the order of 0.1 – 0.2Å. It should be noted that the resolution attained in this approach depends on having two labels at a fixed distance and in a fixed relative orientation. These conditions are not often achieved in SDSL, except in the case of buried, strongly-immobilized sites. Further development to accommodate the distribution of distances and angles normally found with spin labeled cysteine residues is in progress (Hustedt and Beth, 2000).
An important goal in SDSL is the accurate measurement of interspin distances at ambient temperature. This would allow the study of
