
Biomedical EPR Part-B Methodology Instrumentation and Dynamics - Sandra R. Eaton
.pdfSATURATION TRANSFER EPR |
403 |
Feix, J.B., and Klug, C.S. (1998). Site-directed spin labeling of membrane proteins and peptide-membrane interactions. In: Biological Magnetic Resonance, Volume 14: Spin Labeling: The Next Millennium, L.J. Berliner, Ed., Plenum Press, New York, 251-281.
Frye, L.D., and Edidin, M. (1970). The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J. Cell Sci. 7, 319-335.
Fung, L.W., Kalaw, B.O., Hatfield, R.M., and Dias, M.N. (1996). Erythrocyte spectrin maintains its segmental motions on oxidation: a spin-label EPR study. Biophys. J. 70, 841851.
Gaffney, B.J., and Marsh, D. (1998). High-frequency, spin-label EPR of nonaxial lipid ordering and motion in cholesterol-containing membranes. Proc. Natl. Acad. Sci. USA, 95, 12940-12943.
Ge, M., and Freed, J.H. (1993). An electron spin resonance study of interactions between gramicidin A’ and phosphatidylcholine bilayers. Biophys. J. 65, 2106-2123.
Griffith, O.H., and Jost, P.C. (1976). Lipid spin labels in biological membranes. In: Spin Labeling: Theory and Applications, L.J. Berliner, ed., Academic Press, New York, pp. 453-523.
Gwak, S.H., Yu, L., and Yu, C.A. (1986). Spin-label electron paramagnetic resonance and differential scanning calorimetry studies of the interaction between mitochondrial succinate-ubiquinone and ubiquinol-cythchrome c reductases. Biochemistry 25, 76757682.
Hemminga, M.A., and de Jager, P.A. (1989). Saturation transfer spectroscopy of spin labels. Techniques and interpretation of spectra. In: Biological Magnetic Resonance, Vol. 8. Spin Labeling Theory and Applications. L.J. Berliner and J. Reuben, eds., Plenum Press, New York, pp. 131-178.
Herman, J.R., Londo, T.R., Rahman, N.A., and Barisas, B.G. (1992). Normally on photomultiplier gating circuit with reduced post-gate artifacts for use in transient luminescence measurements. Rev. Sci. Instrum. 63, 5454-5458.
Howard, E.C., Lindahl, K.M., Polnaszek, C.F., and Thomas, D.D. (1993) Simulation of saturation transfer electron paramagnetic resonance spectra for rotational motion with restricted angular amplitude. Biophys. J. 64, 581-593.
Hubbell, W.L., Mchaourab, H.S., Altenbach, C., and Lietzow, M.A. (1996). Watching proteins move using site-directed spin labeling. Structure 4, 779-783.
Hubbell, W.L., Cafiso, D.S., and Altenbach, C., (2000). Identifying conformational changes with site-directed spin labeling. Nat. Struct. Biol. 7, 735-739.
Hustedt, E.J., Cobb, C.E., Beth, A.H., and Beechem, J.M. (1993) Measurement of rotational dynamics by the simultaneous non-linear analysis of optical and EPR data, Biophys. J. 64, 614-621.
Hustedt, E.J., and Beth, A.H. (1995). Analysis of saturation transfer electron paramagnetic resonance spectra of a spin-labeled integral membrane protein, band 3, in terms of the uniaxial rotational diffusion model. Biophys. J. 69, 1409-1423.
Hustedt, E.J., and Beth, A.H. (1996). Determination of the orientation of a band 3 affinity spin-label relative to the membrane normal axis of the human erythrocyte. Biochemistry, 35, 6944-6954.
Hustedt, E.J., Smirnov, A.I., Laub, C.F., Cobb, C.E., and Beth, A.H. (1997). Molecular distances from dipolar coupled spin-labels: the global analysis of multifrequency continuous wave electron paramagnetic resonance data. Biophys. J. 74, 1861-1877.
Hustedt, E.J., and Beth, A.H. (1999). Nitroxide spin-spin interactions: Applications to protein structure and dynamics. Annu. Rev. Biophys. Biomol. Struct. 29, 129-153.
404 ALBERT H. BETH AND ERIC J. HUSTEDT
Hustedt, E.J. and Beth, A.H. (2001). Simulation of saturation transfer electron paramagnetic resonance spectra for a restricted uniaxial rotational diffusion model. Biophys. J. 81, 31563165.
Hyde, J.S., Eriksson, L.E.G., and Ehrenberg, A. (1970). EPR relaxation of slowly moving flavin radicals: “Anomalous” saturation. Biochim. Biophys. Acta 222, 688-692.
Hyde, J.S., and Dalton, L.R. (1972). Very slow tumbling spin labels: Adiabatic rapid passage.
Chem. Phys. Lett. 16, 568-572.
Hyde, J.S., and Thomas, D.D. (1974). New EPR methods for the study of very slow motion: Application to spin-labeled hemoglobin. Ann. N. Y. Acad. Sci. 222, 680-692.
Hyde, J.S. (1978). Saturation-transfer spectroscopy. In: Methods in Enzymology, C.H.W. Hirs and S.N. Timasheff, eds., Academic Press, New York, 49G, pp. 480-511.
Hyde, J.S., and Dalton, L.R. (1979). Saturation-transfer spectroscopy. In: Spin Labeling II: Theory and Applications, L.J. Berliner, ed., Academic Press, New York, pp. 1-70.
Hyde, J.S., and Thomas, D.D. (1980). Saturation-transfer spectroscopy. Ann. Rev. Phys. Chem. 31, 293-317.
Hyde, J.S., and Froncisz, W. (1989). Loop gap resonators. In: Advanced EPR: Applications in Biology and Biochemistry, A.J. Hoff, ed., Elsevier, Amsterdam, pp. 227-306.
Hyde, J.S., and Feix, J.B. (1989). Electron-electron double resonance. In: Biological Magnetic Resonance: Volume 8: Spin Labeling, L.J. Berliner and J. Reuben, eds., Plenum Press, New York, pp. 305-337.
Hyde, J.S., Mchaourab, H.S., Camenisch, T.G., Ratke, J.J., Cox, R.W., and Froncisz, W. (1998). Electron paramagnetic resonance detection by time-locked subsampling. Rev. Sci. Instrum. 69, 2622-2628.
Jahnig, F. (1986). The shape of a membrane protein derived from rotational diffusion. Eur. Biophys. J. 14, 63-64
Johnson, M.E., and Hyde, J.S. (1981). 35-GHz (Q-band) saturation transfer electron paramagnetic resonance studies of rotational diffusion. Biochemistry 20, 2875-2880.
Johnson, M.E., Lee, L., and Fung, L.W.-M. (1982a). Models for slow anisotropic rotational diffusion in saturation transfer electron paramagnetic resonance at 9 and 35 GHz.
Biochemistry 21, 4459-4467.
Johnson, M.E., Thiyagarajan, P., Bates, B., and Currie, B. (1982b). A comparison of resolution-enhancement methods in saturation transfer EPR. 15N isotopically substituted spin labels and 35 GHz high-frequency operation. Biophys. J. 37, 553-557.
Kawasaki, K., Yin, J.-J., Subczynski, W.K., Hyde, J.S., and Kusumi, A. (2001). Pulse EPR detection of lipid exchange between lipid-rich raft and bulk domains in the membrane: Methodology development and its application to studies of influenza viral membrane.
Biophys. J. 80, 738-748.
Koppel, D.E., Sheetz, M.P., and Schindler, M. (1981). Matrix control of protein diffusion in biological membranes. Proc. Natl. Acad. Sci. USA 78, 3576-3580.
Lewis, S.M., and Thomas, D.D. (1991). Microsecond rotational dynamics of spin-labeled CaATPase during enzymatic cycling initiated by photolysis of caged ATP. Biochemistry 30, 8331-8339.
Mahaney, J.E., Girard, J.P., and Grisham, C.M. (1990). Saturation transfer EPR measurements of the rotational diffusion of a strongly immobilized ouabain spin label on renal Na,K-ATPase. FEBS Lett. 260, 160-164.
Mahaney, J.E., and Thomas, D.D. (1991). Effects of melittin on molecular dynamics and CaATPase activity in sarcoplasmic reticulum membranes: electron paramagnetic resonance.
Biochemistry 30, 7171-7180.
Mahaney, J.E., and Grisham, C.M. (1992). Effects of ouabain on the rotational dynamics of renal Na,K-ATPase studied by saturation-transfer EPR. Biochemistry 31, 2025-2034.
SATURATION TRANSFER EPR |
405 |
Mangels, M.L., Harper, A.C., Smirnov, A.I., Howard, K.P., and Lorigan, G.A. (2001). Investigating magnetically aligned phospholipids bilayers with EPR spectroscopy at 94 GHz. J. Mang. Reson. 151, 253-259.
Marsh, D., and Henderson, P.J. (2001). Specific spin labeling of the sugar-H(+) symporter, GalP, in cell membranes of Escherichia coli: site mobility and overall rotational diffusion of the protein. Biochim. Biophys. Acta 1510, 464-473.
Martin-Fernandez, M., Clarke, D.T., Tobin, M.J., Jones, S.V., and Jones, G.R. (2002). Preformed oligomeric epidermal growth factor receptors undergo an ectodomain structure change during signaling. Biophys. J. 82, 2415-2427.
Matayoshi, E.D., and Jovin, T.M. (1991). Rotational diffusion of band 3 in erythrocyte membranes. 1. Comparison of ghosts and intact cells. Biochemistry 30, 3527-3538.
Mchaourab, H.S., and Hyde, J.S. (1993). Continuous wave multiquantum electron paramagnetic resonance spectroscopy. III. Theory of intermodulation sidebands. J. Chem. Phys. 98, 1786-1796.
McCalley, R.C., Shimshick, E.J., and McConnell, H.M. (1972). The effect of slow rotational motion on paramagnetic resonance spectra. Chem. Phys. Lett. 13, 115-119.
Mett, R.R., Froncisz, W., and Hyde, J.S. (2001). Axially uniform resonant cavity modes for potential use in electron paramagnetic resonance spectroscopy. Rev. Sci. Instrum. 72, 4188-4200.
Middleton, D.A., Reid, D.G., and Watts, A. (1995). The conformations of a functional spinlabeled derivative of gastric H/K-ATPase investigated by EPR spectroscopy. Biochemistry 34, 7420-7429.
Naqvi, K.R., Gonzalez-Rodriguez, J., Cherry, R.J., and Chapman, D. (1973). Spectroscopic technique for studying protein rotation in membranes. Nature New Biol. 245, 249-251.
Negash, S., Chen, L.T., Bigelow, D.J., and Squier, T.C. (1996). Phosphorylation of phospholamban by cAMP-dependent protein kinase enhances interactions between CaATPase polypeptide chains in cardiac sarcoplasmic reticulum membranes. Biochemistry 35, 11247-11259.
Nigg, E.A., and Cherry, R.J. (1980). Anchorage of a band 3 population at the erythrocyte cytoplasmic membrane surface: Protein rotational diffusion measurements. Proc. Natl. Acad. Sci. USA 77, 4702-4706.
Percival, P.W., and Hyde, J.S, (1976). Saturation recovery measurements of the spin-lattice relaxation times of some nitroxides in solution. J. Magn. Reson. 23, 249-257.
Perkins, R.C., Lionel, T., Robinson, B.H., Dalton, L.A., and Dalton, L.R. (1976). Saturation transfer spectroscopy: Signals sensitive to very slow molecular reorientation. Chem. Phys. 16, 393-404.
Qiu, Z.H., Yu, L., and Yu, C.A. (1992). Spin-label electron paramagnetic resonance and differential scanning calorimetry studies of the interaction between mitochondrial cytochrome c oxidase and adenosine triphosphate synthase complex. Biochemistry 31, 3297-3302.
Robinson, B.H., Dalton, L.R., Dalton, L.A., and Kwiram, A.L. (1974). Fast computer calculation of ESR and nonlinear spin response spectra from the fast motion to the rigid lattice limits. Chem. Phys. Lett. 29, 56-64.
Robinson, B.H., and Dalton, L.R. (1980). Anisotropic rotational diffusion studied by passage saturation transfer electron paramagnetic resonance. J. Chem. Phys. 72, 1312-1324.
Robinson, B.H., and Dalton, L.R. (1981). Approximate methods for the fast computation of EPR and ST-EPR spectra. V. Application of the perturbation approach to the problem of anisotropic motion. Chem. Phys. 54, 253-259.
Robinson, B.H. (1983). Effects of overmodulation on saturation transfer EPR signals. J. Chem. Phys. 78, 2268-2273.
406 ALBERT H. BETH AND ERIC J. HUSTEDT
Robinson, B.H., Thomann, H. Beth, A.H., Fajer, P. and Dalton, L.R. (1985). EPR and advanced EPR studies of biological systems, L.R. Dalton, ed., CRC Press, Boca Raton, Florida, pp. 1-314.
Robinson, B.H., Haas, D.A., and Mailer, C. (1994). Molecular dynamics in liquids: Spinlattice relaxation of nitroxide spin labels. Science 263, 490-493.
Rohrer, M., Prisner, T.F., Brugmann, O., Kass, H., and Spoerner, M. (2001). Structure of the metal-water complex in Ras x GDP studied by high-field EPR spectroscopy and 31P NMR spectroscopy. Biochemistry 40, 1884-1889.
Rousseau, D.L., Guyer, C.A., Beth, A.H., Papayannopoulos, I.A., Wang, B., Wu, R., Mroczkowski, B., and Staros, J.V. (1993). Preparation and characterization of a bifunctionally spin-labeled mutant of murine epidermal growth factor for saturationtransfer electron paramagnetic resonance studies of the growth factor/receptor complex.
Biochemistry 32, 7893-7903.
Saffman, P.G., and Delbruck, M. (1975). Brownian motion in biological membranes. Proc. Natl. Acad. Sci. USA 72, 3111-3113.
Schwarz, D., Pirrwitz, J., Meyer, H.W., Coon, M.J., and Ruckpaul, K. (1990). Membrane topology of microsomal cytochrome P-450: Saturation transfer EPR and freeze-fracture electron microscopy studies. Biochem. Biophys. Res. Comm. 171, 175-181.
Schwarz, D., Chernogolov, A., and Kisselev, P. (1999). Complex formation in vesiclereconstituted mitochondrial cytochrome P450 systems (CYP11A1 and CYP11B1) as evidenced by rotational diffusion experiments using EPR and ST-EPR. Biochemistry 38, 9456-9464.
Sczaniecki, P.B., Hyde, J.S., and Froncisz, W. (1990). Continuous wave multiquantum electron paramagnetic resonance spectroscopy. J. Chem. Phys. 93, 3891-3898.
Singer, S.J. (1972). A fluid lipid-globular protein mosaic model of membrane structure. Ann. N.Y. Acad. Sci. 195, 16-23.
Squier, T.C., and Thomas, D.D. (1988). Relationship between protein rotational dynamics and phosphoenzyme decomposition in the sarcoplasmic reticulum Ca-ATPase. J. Biol. Chem., 263, 9171-9177.
Squier, T.C., Hughes, S.E., and Thomas, D.D. (1988). Rotational dynamics and proteinprotein interactions in the Ca-ATPase mechanism. J. Biol. Chem. 263, 9162-9170.
Smirnov, A.I., Belford, R.L., and Clarkson, R.B. (1998). Comparative spin label spectra at X- band and W-band. In: Spin Labeling: The Next Millenium, L.J. Berliner, ed., Plenum Press, New York, pp. 83-107.
Smirnov, A.I., Smirnova, T.I., and Morse, P.D. (1995). Very high frequency electron paramagnetic resonance of 2,2,6,6-tetramethyl-1-piperidinyloxy in 1,2-dipalmitoyl-sn- glycero-3-phosphaticylcholine liposomes: Partitioning and molecular dynamics. Biophys. J. 68, 2350-2360.
Stein, R.A., Hustedt, E.J., Staros, J.V., and Beth, A.H. (2002). Rotational dynamics of the epidermal growth factor receptor. Biochemistry 41, 1957-1964.
Stryer, L. (1978). Fluorescence energy transfer as a spectroscopic ruler. Ann. Rev. Biochem. 47, 819-846.
Szabo, A. (1984). Theory of fluorescence depolarization in macromolecules and membranes.
J. Chem. Phys. 81, 150-167.
Thomas, D.D., and McConnell, H.M. (1974). Calculation of paramagnetic resonance spectra sensitive to very slow rotational motion. Chem. Phys. Lett. 25, 470-475.
Thomas, D.D., Dalton, L.R., and Hyde, J.S. (1976). J. Chem. Phys. 65, 3006-3024.
Thomas, D.D., Eads, T.M., Barnett, V.A., Lindahl, K.M., Momont, D.A., and Squier, T.C. (1985). Saturation transfer EPR and triplet anisotropy: Complementary techniques for the
SATURATION TRANSFER EPR |
407 |
study of microsecond rotational dynamics. In: Molecular Biological Systems, P. Bayley and R. Dale, eds., Academic Press, New York, pp. 239-257.
Thomas, D.D. (1985). Saturation transfer EPR studies of microsecond rotational motions in biological membranes. In: The Enzymes of Biological Systems, A.N. Martonosi, ed., Plenum, New York, pp. 287-312.
Wahl, Ph. (1975) Fluorescence anisotropy of chromophores rotating between two reflecting barriers. Chem. Phys. 7, 210-219.
Webb, W.W. (1981). Luminescence measurements of macromolecular mobility. Ann. N. Y. Acad. Sci. 366, 300-314.
Weber, G. (1971). Theory of fluorescence depolarization by anisotropic Brownian rotations. Discontinuous distribution approach. J. Chem. Phys. 55, 2399-2411.
Wolkers, W.F., Spruijt, R.B., Kaan, A., Konings, R.N., and Hemminga, M.A. (1997). Conventional and saturation-transfer EPR of spin-labeled mutant bacteriophage M13 coat protein in phospholipids bilayers. Biochim. Biophs. Acta 1327, 5-16.
Zacharias, D.A., Violin, J.D., Newton, A.C., and Tsien, R.Y. (2002). Partitioning of lipidmodified monomeric GFP’s into membrane microdomains of live cells. Science 296, 913916.
410 |
JAMES S. HYDE |
From its earliest days, the underlying technology was based on military developments in radar. In recent years, computers, cellular telephone technology and advances in digital devices have become increasingly important in contributing to the technological foundation of EPR spectrometers.
This reality – that our field is relatively small and dependent to a considerable degree on technology from larger scale development activities
– places constraints on future progress of the field. The fundamental technological event of our times is rapid increase in computing power and the performance of associated digital and mass storage devices. Our future, from a technological perspective, will be based on this fact. Advances in EPR digital detection, in data capture and storage as well as in use of advanced signal processing methods are discussed in the chapter on digital detection (see Ch. 7).
Even though EPR instrumentation is strongly dependent on technology developed in other fields, there are areas where the special constraints of EPR have led to significant technological advances. Some of these are discussed below.
2.RESONATORS
Basic contributions to EPR technology that have been developed from within the EPR discipline include resonator development. The requirements of sample access, variable microwave coupling, resonators free from impurities, wall penetrability by high frequency field modulation, temperature control, etc. place constraints on resonator design that we ourselves must face – there is little in the way of technology to borrow from other fields. It is appropriate for the EPR instrumental futurist to predict advances in those specific areas where we control the technology and an attempt is made here.
The Varian multipurpose cavity oscillating in the rectangular 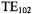 mode served as the default EPR resonator for many years. See Rempel et al. (1964) and Hyde (1995) for details on the design. This structure was designed to enable a number of specialized EPR experiments as follows: i) light irradiation, ii) dewar insert for flowing temperature controlled gas, iii) dewar insert for liquid nitrogen, iv) so-called “flat cells” for aqueous samples, v) flat cells for tissue samples, vi) a mixing chamber geometry for stopped and continuous flow EPR, and vii) an electrochemical cell. A collet system was developed to support the dewars, flat cells, and sample tubes of various sizes. Two cavity bodies were bolted together to form the dual sample cavity (Hyde, 1965a) oscillating in the
mode served as the default EPR resonator for many years. See Rempel et al. (1964) and Hyde (1995) for details on the design. This structure was designed to enable a number of specialized EPR experiments as follows: i) light irradiation, ii) dewar insert for flowing temperature controlled gas, iii) dewar insert for liquid nitrogen, iv) so-called “flat cells” for aqueous samples, v) flat cells for tissue samples, vi) a mixing chamber geometry for stopped and continuous flow EPR, and vii) an electrochemical cell. A collet system was developed to support the dewars, flat cells, and sample tubes of various sizes. Two cavity bodies were bolted together to form the dual sample cavity (Hyde, 1965a) oscillating in the  mode in order to
mode in order to
TRENDS IN EPR TECHNOLOGY |
411 |
examine a reference sample and a sample of interest simultaneously. A top coupled section was added (Piette et al., 1962) to form a 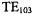 cavity that permitted simultaneous optical absorption and EPR experiments on a sample. The demountable sidewall construction permitted disassembly and cleaning. Dr. Robert Rempel was the leader of this initiative with substantial contributions by Dr. Lawrence Piette, both of whom were Varian scientists. This outpouring of technology provided the basis for transfer of EPR spectroscopy from its original base in physics into chemistry and biology.
cavity that permitted simultaneous optical absorption and EPR experiments on a sample. The demountable sidewall construction permitted disassembly and cleaning. Dr. Robert Rempel was the leader of this initiative with substantial contributions by Dr. Lawrence Piette, both of whom were Varian scientists. This outpouring of technology provided the basis for transfer of EPR spectroscopy from its original base in physics into chemistry and biology.
A number of special purpose X-band cavities were developed commercially. A list of some of these includes: cylindrical 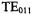 “wirewound” cavity (Hyde et al., 1965b, 1966) for enhanced sensitivity in certain classes of samples, ENDOR cavities, cavities for use at cryogenic temperatures,
“wirewound” cavity (Hyde et al., 1965b, 1966) for enhanced sensitivity in certain classes of samples, ENDOR cavities, cavities for use at cryogenic temperatures, 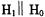 cavity, ELDOR cavities and the
cavity, ELDOR cavities and the 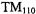 cavity for aqueous samples (Hyde, 1975).
cavity for aqueous samples (Hyde, 1975).
This rich array of X-band resonators is the primary reason that this microwave frequency remains dominant in EPR spectroscopy. Nothing comparable exists at either higher or lower microwave frequencies. This is a significant opportunity for future technological development. Certainly all of these X-band capabilities could be replicated at Q-band (35 GHz) and S- band (ca 3 GHz), which would greatly strengthen the concept of “multifrequency EPR.”
In 1965, the author introduced a system of classification for EPR samples from the perspective of sensitivity and optimum microwave configuration: namely, eight classes of samples depending on yes or no answers to three questions: i) Does it saturate? ii) Is it limited in size or availability? iii) Does it exhibit substantial dielectric loss (Varian Associates, 1965)? Of course, real samples may not correspond to one of these classes, but would lie in some intermediate category. Nevertheless, this classification system has proven helpful in thinking about resonator design in EPR spectroscopy. To a certain degree, it lies at the heart of the development of loop-gap resonators (LGR) for use in EPR spectroscopy, largely by W. Froncisz and the author (See Froncisz and Hyde, 1982; Hyde and Froncisz, 1989; and ch. 2 in the present volume). This class of resonators fundamentally improves sensitivity for those four classes of samples that correspond to a YES answer concerning question ii): Is the sample limited in size or availability? The benefits with respect to sensitivity can be quite substantial. The X-band LGR with 1 mm diameter sample access hole is one of the enabling technologies for the development of site directed spin labelling. The LGR is also a central technology for in vivo small animal imaging and spectroscopy (see ch 9 and ch. 11 in volume 23.)
Simulation of electromagnetic fields in microwave cavity resonators using finite-element computer-driven solutions of Maxwell’s equations holds great
412 JAMES S. HYDE
promise for future development of EPR resonators. The author is aware of six papers in the literature where finite element modeling of electromagnetic fields was employed in a context of microwave resonators for EPR use. The earliest papers were from A. Schweiger’s group in Zurich (Pfenninger et al., 1988; Forrer et al., 1996). In Pfenninger et al. (1988) the program known as MAFIA (Solution of MAxwell’s equation by the Finite Integration Algorithm) was used as an aid to development of bridged loop gap resonators. The work of this group in this area was updated in Forrer et al., 1996. Also in that year, the MCW group used the Hewlett-Packard version of High Frequency Structure Simulator, HFSS, to develop a bimodal loop gap resonator (Piasecki et al., 1996).
Recently, the author and his colleagues wrote a series of three papers on a new class of microwave resonators that we chose to call uniform field (UF) cavities (Mett et al., 2001; Anderson et al., 2002; Hyde et al., 2002). Transverse magnetic field (TM) modes exist, that exhibit uniformity of the fields along the axis that is perpendicular to the transverse field. This is because Maxwell’s equations allow magnetic fields that are tangential to conducting end walls to exist. The familiar 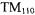 cylindrical cavity widely used in EPR for aqueous samples is an example of such a structure. However, previous to these three papers there were no known transverse electric field (TE) modes that exhibited this property of uniformity of the fields along the perpendicular axis. This is because components of the microwave electric field tangential to conducting end walls cannot exist. The UF modes described in these three papers consisted of a central section where the fields were precisely uniform, with end sections designed to satisfy the boundary conditions at the end walls.
cylindrical cavity widely used in EPR for aqueous samples is an example of such a structure. However, previous to these three papers there were no known transverse electric field (TE) modes that exhibited this property of uniformity of the fields along the perpendicular axis. This is because components of the microwave electric field tangential to conducting end walls cannot exist. The UF modes described in these three papers consisted of a central section where the fields were precisely uniform, with end sections designed to satisfy the boundary conditions at the end walls.
These three papers made extensive use of high frequency structure simulation (HFSS) finite element software. UF resonators were, in fact, discovered using HFSS. We were working on an unrelated problem and noticed some unusual microwave field patterns that we did not understand. When the structures were built, they worked exactly as designed. Although they were largely theoretical, experimental “proof-of-principle” data were presented. Design of microwave coupling structures and sample entrance structures (so-called “stacks”) for these prototypical resonators was also carried out using HFSS.
The HP HFSS software, which is no longer available, operated in the socalled driven mode. We found it very difficult to use for microwave cavity development because every change in the structure required a tedious hunt for the slightly shifted resonant frequency. When Hewlett-Packard dropped out of this business, we shifted to Ansoft HFSS (Ansoft Corp., Pittsburgh, PA). This software incorporates both the driven mode and the 3D modal frequency or “eigenmode” solution method (Brauer, 1997). This method is
TRENDS IN EPR TECHNOLOGY |
413 |
also available in MAFIA. We find that this method is extremely useful for EPR resonator development (Mett et al., 2001; Anderson et al., 2002; Hyde et al., 2002). Since resonators are characterized by a single resonant mode of interest, use of the eigenmode method permits focus only on that mode; a frequency sweep is not needed. Changes to the structure can be made and the resulting effect seen on the fields, resonance frequency and Q-value without having to track the changes through a frequency scan. Second, using the eigenmode method, we can focus on the design of the resonator without introducing a coupling structure. Mechanical drawing time and computation time are each reduced by one to two orders of magnitude due to the increased symmetry of the structure. Introduction of a coupling structure as well as sample access stacks is straightforward in the final stages of a design. We have also used the software to calculate the effects of sample support structures, dewar inserts, and the effect of the dielectric properties of the sample on the electromagnetic field distribution. The resonator efficiency parameter  (Hyde and Froncisz, 1989) can be calculated, which permits an estimate of EPR sensitivity. Currently we run this code on a Compaq W8000 workstation with dual Xeon 1.7 GHz processors with 2 GB of RAM.
(Hyde and Froncisz, 1989) can be calculated, which permits an estimate of EPR sensitivity. Currently we run this code on a Compaq W8000 workstation with dual Xeon 1.7 GHz processors with 2 GB of RAM.
In another cavity development initiative, we gave ourselves the goal of finding a way to examine a normal X-band sample in a 3 mm i.d., 4 mm o.d. quartz sample tube at Q-band. The problem was to determine whether the microwave sample access stacks would be beyond cutoff. As the sample diameter increases, the stack, viewed as a cylindrical waveguide propagating in either the cylindrical  or cylindrical
or cylindrical 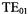 modes will first become evanescent and eventually radiate power into space. It was found that practical structures for this purpose can be constructed providing that the stack is sufficiently long.
modes will first become evanescent and eventually radiate power into space. It was found that practical structures for this purpose can be constructed providing that the stack is sufficiently long.
Ansoft offers another software package known as Maxwell 3D that is well configured for computation of the 100 kHz field modulation patterns and eddy currents in surrounding metallic structures. Four approaches to the design of field modulation assemblies for use in EPR can be identified from the existing literature: i) Use of electrically thin walls – less than one skin depth at 100 kHz but many skin depths at the microwave frequency. ii) The wirewound technique introduced by the author for use with the cylindrical
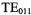 mode (Hyde, 1965, 1966, and Fig. 1). All microwave currents must be substantially parallel to the wires. iii) Partially cut-through slots for use with loop gap resonators (Fig. 2). iv) Direct insertion into the resonator of wires or rods that carry field modulation current. In addition to modeling of fields produced by modulation coils, Maxwell 3D is well suited for modeling of fields in a context of ENDOR and RF coils. It is apparent that these various
mode (Hyde, 1965, 1966, and Fig. 1). All microwave currents must be substantially parallel to the wires. iii) Partially cut-through slots for use with loop gap resonators (Fig. 2). iv) Direct insertion into the resonator of wires or rods that carry field modulation current. In addition to modeling of fields produced by modulation coils, Maxwell 3D is well suited for modeling of fields in a context of ENDOR and RF coils. It is apparent that these various
