
Biomedical EPR Part-B Methodology Instrumentation and Dynamics - Sandra R. Eaton
.pdfMEASUREMENT OF DISTANCES BETWEEN ELECTRON SPINS |
231 |
MHz. For the DEER experiments  typically is selected such that there is minimal overlap between the bandwidth of spins observed at
typically is selected such that there is minimal overlap between the bandwidth of spins observed at 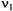 and pumped at
and pumped at 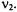 To permit distinctions between intramolecular and intermolecular electron-electron spin-spin interaction, the spin-labeled peptide was diluted with varying ratios of unlabeled protein and the total peptide concentration was varied. At high dilutions with unlabeled protein, characteristic DEER oscillations were observed that correspond to an interspin distance of 15.7 Å, which is consistent with a
To permit distinctions between intramolecular and intermolecular electron-electron spin-spin interaction, the spin-labeled peptide was diluted with varying ratios of unlabeled protein and the total peptide concentration was varied. At high dilutions with unlabeled protein, characteristic DEER oscillations were observed that correspond to an interspin distance of 15.7 Å, which is consistent with a 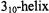 conformation of the peptide. Comparison of the amplitudes of rapidly decaying and oscillating contributions to the DEER signal for peptide aggregates indicated that about 19% of the peptides adopted the conformation with this interspin distance. The DEER results were consistent with a model in which four of the amphiphilic peptides form a structured aggregate.
conformation of the peptide. Comparison of the amplitudes of rapidly decaying and oscillating contributions to the DEER signal for peptide aggregates indicated that about 19% of the peptides adopted the conformation with this interspin distance. The DEER results were consistent with a model in which four of the amphiphilic peptides form a structured aggregate.
6.3Characterization of Clusters in Ionomers (Pannier et al., 2000)
The 4-pulse DEER experiment was used to characterize the size of clusters and distance between clusters in poly(isoprene) polymers with sulfonate end groups and for poly(styrene)-poly(isoprene) diblock polymers with sulfonate end groups on the poly(isoprene) chains. The 4-pulse DEER experiment was important for this application because the absence of a deadtime permitted characterization of the short interspin distances within the clusters. The potassium salt of 4-carboxy-tempo (K-tempo) was added in a ratio of two spin labels per 15 ionic end groups. The immobilization of the spin label shown by CW EPR indicated that the charged nitroxyls were associated with the charged endgroups of the polymers. The DEER experiments were performed at X-band at 15 K on a Bruker E380E spectrometer using an ENDOR resonator that was overcoupled to Q ~ 100 to give a bandwidth that was large enough to accommodate two microwave frequencies that differed by 50 – 70 MHz. The DEER signals for poly(isoprene) exhibited three components that could be modeled by two Gaussian distance distributions with mean values of 15 – 20 Å and 40 to 70 Å, and a uniformly distributed background. The 15 – 20 Å distribution was attributed to interacting spins within a cluster, which therefore defined the size of the clusters. The distribution with longer distances defined the intercluster distances. The intercluster distance was consistent with prior results from small angle X-ray scattering (SAXS) and the distance within a cluster was consistent with molecular modeling calculations. The DEER method also gave plausible results for the diblock polymer, for which attempts to measure the intercluster distance by SAXS were unsuccessful.
232 |
SANDRA S. EATON AND GARETH R. EATON |
This study demonstrates the utility of 4-pulse DEER in characterizing heterogeneous systems.
6.4Separation between spins radicals pairs of the Photosystem I (Bittl and Zech, 2001)
Modulation of the out-of-phase echo is a powerful technique to
determine the distance within the charge-separated pair,
Detection of the spin echo is synchronized with laser pulses that generate the radical pair. Subsequent X-ray crystallographic results validated the distance of 28.4 Å obtained by this technique. This is a convenient technique for monitoring structural changes that occur (or do not occur) due to sitedirected mutagenesis or quinone substitution. A detailed discussion of this technique is given in Dzuba and Hoff (2000).
7.RECENT EXAMPLES FOR DISTANCES BETWEEN A RAPIDLY RELAXING AND A SLOWLY RELAXING SPIN
7.1Spin-Labeled High-Spin Metmyoglobin Studied by Saturation Recovery (Zhou et al., 2000)
Calculations of the distance between a rapidly relaxing metal ion and a neighboring organic radical based on enhancement of spin lattice relaxation rates have used the Bloembergen equation and its modifications (Eaton and Eaton, 2000c; Lakshmi and Brudvig, 2000). However, the Bloembergen equation was derived for nuclear spins, so the zero-field splitting (ZFS) that is present for metals with S > ½ was not included. An approach analogous to the derivation of the Bloembergen equation was performed, including the additional energy splittings that arise from the zero-field splitting, for a metal with S = 5/2 and ZFS much greater than the EPR quantum. An additional complication arises for Kramers’ ions with S > ½ and large ZFS in that the observable EPR transitions are between the 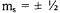 spin states. Since the metal spin-lattice relaxation rates are expected to be different for different values of
spin states. Since the metal spin-lattice relaxation rates are expected to be different for different values of 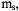 measurement of relaxation rates for the observed transitions will not be representative of all the metal spin states interacting with the slowly relaxing spin. The approach that was taken in this study was to fix the interspin distance for one spin-labeled high-spin met-myoglobin variant at the value obtained for the low-spin analog and treat the metal relaxation rate as the adjustable parameter in simulating long-pulse
measurement of relaxation rates for the observed transitions will not be representative of all the metal spin states interacting with the slowly relaxing spin. The approach that was taken in this study was to fix the interspin distance for one spin-labeled high-spin met-myoglobin variant at the value obtained for the low-spin analog and treat the metal relaxation rate as the adjustable parameter in simulating long-pulse
MEASUREMENT OF DISTANCES BETWEEN ELECTRON SPINS |
233 |
saturation recovery curves for the interacting spin label. The resulting values of the iron relaxation rates were systematically faster than values obtained by simulation of the temperature dependent contribution to the CW lineshapes for the high-spin Fe(III) EPR spectra. When these calculated iron relaxation rates were used to obtain interspin distances by analysis of longpulse saturation recovery curves for a set of 12 spin-labeled metmyoglobin variants, preliminary results indicate reasonable agreement in distances between high-spin and low-spin variants for distances between about 16 and 30 Å (Ulyanov et al., unpublished).
7.2Spin-Labeled High-Spin Metmyoglobin Studied by Spin Echo (Ulyanov et al., unpublished)
When the relaxation rate for the rapidly relaxing spin is comparable to the magnitude of the electron-electron dipolar coupling in frequency units, the metal relaxation is an effective dephasing mechanism for the two-pulse spin echo of the slowly relaxing center (Rakowsky et al., 1998; Eaton and Eaton, 2000c). These dramatic effects on spin echo decays have been demonstrated (Seiter et al., 1998), but the analysis of the full decay curves to determine the interspin distance is tedious. The enhanced rate of dephasing results in decreased echo intensity at each point along the decay curve. For a fixed timing of the two-pulse echo sequence, as the rate of relaxation for the fast relaxing spin increases with increasing temperature, the echo intensity for the slowly relaxing spin goes through a minimum. Calculations predict that the minimum echo intensity observed as a function of temperature decreases as the interspin distance decreases (Eaton and Eaton, 2000c). For a series of spin-labeled variants of low-spin cyano-metmyoglobin, preliminary results show that the minimum nitroxyl echo intensity correlates well with the interspin distance obtained by analysis of long-pulse saturation recovery curves (Figure 1). The advantage of the spin echo measurements relative to the saturation recovery measurements is that values of the iron relaxation rates as a function of temperature are not required to calculate the interspin distance from the spin echo intensity. This method shows considerable promise for distance measurements.

234 |
SANDRA S. EATON AND GARETH R. EATON |
Figure 1. Correlation between minimum nitroxyl two-pulse spin echo intensity as a function of temperature for a pulse spacing of 200 ns, and interspin distance determined by analysis of long-pulse saturation recovery curves (Ulyanov et al., unpublished).
8.PROGNOSIS
Interspin distances in the range of 20 to about 50 Å determined by pulsed EPR provide important constraints on structural models in a particularly useful distance range. As computational techniques become more powerful in predicting secondary structure, it may become possible to distinguish between postulated tertiary structures based on a few long-distance constraints. One area in which we expect many applications of EPR distance measurements is assemblies of subunits. For example, with appropriate spin labeling, it will be possible to determine whether one or another portion of a

MEASUREMENT OF DISTANCES BETWEEN ELECTRON SPINS |
235 |
molecule associates with a particular region of another molecule in an assembly. Similarly, structural changes could be measured during successive steps in a biological process.
9.ACKNOWLEDGMENTS
Our studies of distances in biomolecules are supported by NIH grant GM21156.
10.REFERENCES
Berliner, L. J., Eaton, S. S., and Eaton, G. R., eds. (2000). Distance Measurements in Biological Systems by EPR, Biol. Magn. Reson, 19.
Bittl, R., and Zech, S. G. (2001). Pulsed EPR Spectroscopy On Short-Lived Intermediates In Photosystem I, Biochim. Biophys. Acta 1507, 194-211.
Borbat, P. P. and Freed, J. H. (2000). Double-Quantum ESE and Distance Measurements Distance Measurements in Biological Systems by EPR, L. J. Berliner, S. S. Eaton, and G. R. Eaton, eds., Biol. Magn. Reson. 19, 383-459.
Borbat, P. P., Mchaourab, H. S., and Freed, J. H. (2002). Protein Structure Determination Using Long-Distance Constraints from Double-Quantum Coherence ESR: Study of T4 Lysozyme, J. Am. Chem. Soc. 124, 5304-5314.
Coffman, R. E., and Buettner, G. R. (1979). A Limit Function for Long-Range Ferromagnetic and Antiferromagnetic Superexchange. J. Phys. Chem. 83, 2387-2392.
Dzuba, S. A. and Hoff, A. J. (2000). Photo-Induced Radical Pairs Investigated using Out-of- Phase Electron Spin Echo, Biol. Magn. Reson. 19, 569-596.
Dzuba, S. A., and Kawamori, A. (1993). Selective Hole Burning: Spectral Diffusion and Dipolar Broadening, Concepts in Magnetic Resonance 8, 49-61.
Eaton, G. R. and Eaton, S. S. (1998). EPR Studies of Long-Range Intramolecular ElectronElectron Exchange Interaction, Accts. Chem. Res. 21, 107-113.
Eaton, S. S. and Eaton, G. R. (2000a). Distance Measurements by CW and Pulsed EPR in Distance Measurements in Biological Systems by EPR, L. J. Berliner, S. S. Eaton, and G. R. Eaton, eds., Biol. Magn. Reson. 19, 2-21.
Eaton, S. S. and Eaton, G. R. (2000b). Relaxation Times of Organic Radicals and Transition Metal Ions in Distance Measurements in Biological Systems by EPR, L. J. Berliner, S. S.
Eaton, and G. R. Eaton, eds., Biol. Magn. Reson. 19, 29-154. |
|
|
Eaton, S. S., and Eaton, G. R. (2000c). Determination of Distances Based on |
and |
in |
Distance Measurements in Biological Systems by EPR, L. J. Berliner, S. S. Eaton, and G. R. Eaton, eds., Biol. Magn. Reson. 19, 347-381.
Harbridge, J. R., Eaton, S. S., Eaton, G. R. (2003). Electron Spin-Lattice Relaxation Processes of Radicals in Irradiated Crystalline Organic Compounds, J. Phys. Chem. A 107, 598-610.
Jeschke, G. (2002) Determination of the Nanostructure of Polymer Materials by Electron Paramagnetic Resonance Spectroscopy, Macromol. Rapid Commun. 23, 227-246.
Jeschke, G., Pannier, M., Spiess, H. W. (2000a). Double Electron-Electron Resonance in Distance Measurements in Biological Systems by EPR, L. J. Berliner, S. S. Eaton, and G. R. Eaton, eds., Biol. Magn. Reson. 19, 493-512.
236 SANDRA S. EATON AND GARETH R. EATON
Jeschke, G., Pannier, M., Godt, A., and Spiess, H. W. (2000b). Dipolar Spectroscopy and Spin Alignment in Electron Paramagnetic Resonance, Chem. Phys. Lett. 331, 243-252.
Jeschke, G., Koch, A., Jonas, U., Godt, A. (2002). Direct Conversion of EPR Dipolar Time Evolution Data to Dipolar Distances, J. Magn. Reson. 155, 72-82.
Lakshmi, K. V., and Brudvig, G. W. (2000). Electron Paramagnetic Resonance Distance Measurements in Photosynthetic Reaction Centers in Distance Measurements in Biological Systems by EPR, L. J. Berliner, S. S. Eaton, and G. R. Eaton, eds., Biol. Magn. Reson. 19, 493-512.
Lu, Y., and Valentine, J. S. (1997). Engineering Metal-Binding Sites in Proteins, Curr. Opin. Struct. Biol. 7, 495-500.
Lu, Y. S., Berg, S. M., and Pfister, T. D. (2001). Engineering Novel Metalloproteins: Design of Metal-Binding Sites into Native Protein Scaffolds, Chem. Rev. 101, 3047-3080.
Luckhurst, G. R. (1976). Biradicals as Spin Probes in Spin Labeling: Theory and Applications, L. J. Berliner, ed., Academic Press, N. Y., ch. 4.
Milov, A. D., Maryasov, A. G., and Tsvetkov, Y. D. (1998). Pulsed Electron Double Resonance (PELDOR) and its Applications in Free Radicals Research. Appl. Magn. Reson. 15, 107-143.
Milov, A. D., Tsvetkov, Yu. D., Formaggio, F., Crisma, M., Toniolo, C., Raap, J. (2001). The Secondary Structure of a Membrane-Modifying Peptide in a Supramolecular Assembly Studied by PELDOR and CW-ESR Spectroscopies, J. Am. Chem. Soc. 123, 3784-3789.
Pannier, M., Schädler, V., Schöps, M., Wiesner, U., Jeschke, G., Spiess, W. (2000). Determination of Ion Cluster Size and Cluster-to-Cluster Distances in Ionomers by FourPulse Double Electron Electron Resonance Spectroscopy, Macromol. 33, 7812-7818.
Rakowsky, M. H., Zecevic, A., Eaton, G. R., and Eaton, S. S. (1998). Determination of HighSpin Iron(III)-Nitroxyl Distances in Spin-Labeled Porphyrins by Time-Domain EPR. J. Magn. Reson. 131, 97-110.
Ratsimring, A. (2000). “2+1” Pulse Sequence as Applied for Distance and Spatial Distribution Measurements of Paramagnetic Centers, in Distance Measurements in Biological Systems by EPR, L. J. Berliner, S. S. Eaton, and G. R. Eaton, eds., Biol. Magn. Reson. 19, 461-491.
Regan, L. (1993). The Design of Metal-Binding Sites in Proteins. Ann. Rev. Biophys. Biomol. Struct. 22, 257-281.
Seiter, M., Budker, V., Du, J.-L, Eaton, G. R., and Eaton, S. S. (1998). Interspin Distances Determined by Time Domain EPR of Spin-Labeled High-Spin Methemoglobin. Inorg. Chim. Acta 273, 354-366.
Ulyanov, D., Bowler, B. E., Eaton, G. R., and Eaton, S. S. (to be published).
Voss, J. L., Hubbell, W. L., and Kaback, H. R. (1995). Distance Determination in Proteins using Designed Metal Binding Sites and Site-Directed Spin Labeling: Application to the Lactose Permease of Escherichia Coli, Biochemistry 34, 6272-6277.
Zhou, Y., Bowler, B. E., Lynch, K., Eaton, S. S. and Eaton, G. R. (2000). Interspin Distances in Spin-Labeled Metmyoglobin Variants Determined by Saturation Recovery EPR. Biophys. J. 79, 1039-1052.
II
Motion, Proteins, and Membranes
Chapter 9
ESR and Molecular Dynamics
Jack H. Freed
Department of Chemistry, and Chemical Biology, Baker Laborator, Cornell University, Ithaca, New York 14853-1301
Abstract: The development of ESR for the study of spin-relaxation and molecular dynamics of organic radicals and spin labels in fluids is reviewed from a historical perspective.
1.MOTIONAL NARROWING AND ORGANIC RADICALS
My interest in electron spin relaxation and molecular dynamics began when I was a graduate student with George Fraenkel at Columbia University from 1958-1962. His laboratory was teeming with interest and activity in the area of spin-relaxation, mainly of organic free radicals in liquid solution. Fraenkel had developed a new theory (Stephen and Fraenkel, 1960) which could successfully account for the fact that the measured 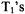 from each hyperfine line of semiquinone spectra obtained in his lab were different in magnitude (Schreurs and Fraenkel 1961). Kivelson was completing his theory of unsaturated linewidths, (Kivelson, 1960) that he developed from the seminal Kubo and Tomita theory of lineshapes, (Kubo and Tomita 1954). The Stephen-Fraenkel theory deriving more from the WangnessBloch (1953) and Redfield (1957) theories, (more commonly known as Redfield theory today) also incorporated components of Kubo and Tomita theory. In fact, the Stephen-Fraenkel theory of ESR (electron-spin resonance) saturation and the Kivelson theory of unsaturated linewidths were complementary in the insight and understanding they provided into spin-relaxation of organic radicals in solution.
from each hyperfine line of semiquinone spectra obtained in his lab were different in magnitude (Schreurs and Fraenkel 1961). Kivelson was completing his theory of unsaturated linewidths, (Kivelson, 1960) that he developed from the seminal Kubo and Tomita theory of lineshapes, (Kubo and Tomita 1954). The Stephen-Fraenkel theory deriving more from the WangnessBloch (1953) and Redfield (1957) theories, (more commonly known as Redfield theory today) also incorporated components of Kubo and Tomita theory. In fact, the Stephen-Fraenkel theory of ESR (electron-spin resonance) saturation and the Kivelson theory of unsaturated linewidths were complementary in the insight and understanding they provided into spin-relaxation of organic radicals in solution.
239
240 |
JACK H. FREED |
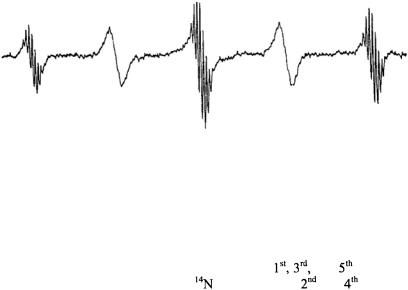
Figure 1. ESR spectrum of p-dinitrotetramethylbenzene radical at 20°C showing the alternating linewidth effect. (Magnetic field increases to the right). From Freed and Fraenkel (1962).
In Fraenkel’s lab my interest was piqued by the “anomalous alternating linewidth” effect. The electrochemically generated ESR spectrum of the p- dinitrotetramethylbenzene anion showed features that had not been seen
before: well-resolved proton shfs appeared on the |
and |
lines of the |
|
hf splitting from the two equivalent |
nuclei; but the |
and |
lines were |
so broad that the proton shfs was completely masked (see Fig. 1). Neither the Kivelson theory of linewidths nor the Stephen-Fraenkel theory of spinrelaxation could explain such a phenomenon. I found that the problem with the earlier theories rested in their improper treatment of multiple or degenerate hf lines which are commonplace for organic radicals. A simple extension of the Kubo-Tomita theory led to the viewpoint that such a multiple hf line must be an “average Lorentzian”. I was able to show rigorously from the Redfield theory, that such a multiple hf line must, in general, be a superposition of Lorentzians. For the specific case of the alternating-linewidths of p-dinitrotetramethylbenzene, a particular molecular motional model was needed to complete the explanation. The new theory required out-of-phase correlation of the two 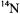 hfs, which are assumed to be fluctuating in time, (Freed and Fraenkel, 1962). (One such model would be a rotation of one nitro-group into the benzene plane, thereby increasing its spin-density, while the other is forced to rotate out of the plane, thereby decreasing its spin density, possibly assisted by counterion motions). Such a process would broaden all the hf components except for those arising from nuclear spin configurations in which the two
hfs, which are assumed to be fluctuating in time, (Freed and Fraenkel, 1962). (One such model would be a rotation of one nitro-group into the benzene plane, thereby increasing its spin-density, while the other is forced to rotate out of the plane, thereby decreasing its spin density, possibly assisted by counterion motions). Such a process would broaden all the hf components except for those arising from nuclear spin configurations in which the two 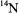 nuclear spin quantum numbers were equal. Work by Bolton and Carrington (1962) at that time on alternating linewidths in durosemiquinone using modified Bloch equations was also consistent with this analysis.
nuclear spin quantum numbers were equal. Work by Bolton and Carrington (1962) at that time on alternating linewidths in durosemiquinone using modified Bloch equations was also consistent with this analysis.
As is often the case in science, the resolution of an “anomaly” led to the formulation of a more generally inclusive theory, in this case the theory of linewidths for organic free radicals. It is, with pleasure, that I note this
ESR AND MOLECULAR DYNAMICS |
241 |
theory, published in 1963, (Freed and Fraenkel, 1963) is still accepted today as valid for spectra in the motional narrowing regime. The important improvements to the Freed-Fraenkel theory since then have largely to do with the incorporation of more precise and detailed models of the molecular dynamics into the formulation. One important example of this, was the incorporation of Perrin’s model (Perrin, 1934) of anisotropic rotational diffusion into the linewidth theory, and its illustration by reinterpreting a linewidth study on p-dinitrobenzene, (Freed, 1964). This work made clear the utility of ESR for the study of molecular dynamics in liquids.
Another improvement was Fraenkel’s (1965) extension of the linewidth theory to include the effects of dynamic frequency shifts, which accompany the fast motional linewidths but are smaller except in special cases, such as for the alternating linewidth effect.
2.DOUBLE RESONANCE AND MOLECULAR DYNAMICS
In 1964, Jim Hyde and Gus Maki first observed ENDOR for organic radicals in liquids, (Hyde and Maki, 1964) (Note ENDOR stands for electron-nuclear double resonance, wherein the ESR signal is partially saturated and the nuclear spins on the radical are irradiated at their NMR frequency). At that time there was no theory for explaining why ENDOR can occur in liquids, and the reason for their successful observation was a mystery. Did it involve spin relaxation and therefore would be relevant for studies of molecular dynamics? Thus it seemed appropriate at that time to undertake a reformulation and generalization of the theory of ESR saturation by analogy to what Fraenkel and I had done with ESR linewidths, and to see if a complete theory would allow for a satisfactory explanation of the HydeMaki experiment. This ultimately led to a very general theory of ESR saturation and double resonance which appeared in 1965, (Freed, 1965). This theory showed that any ENDOR effects must be small. [At that time, Jim Hyde came to Cornell to give a lecture. I explained to Jim how I had looked everywhere in “spin-relaxation space”, and I still couldn’t find effects of more than about a percent. Jim promptly assured me that was about the magnitude of the effects he was seeing! By chopping the NMR frequency, and detecting at the chopping frequency, Jim could get just the difference signal due to the ENDOR]. This formulation and its later extensions have served as the basis of interpreting ESR saturation and ENDOR experiments for motionally-narrowed spectra up to today, (Dorio and Freed, 1979; Kurreck et al, 1988; Möbius et al, 1989).
