
Reference_01_08_2014_165529
.pdf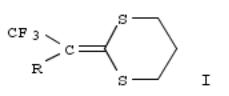
SciFinder® |
Page 191 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
306. 2-(2,2,2-Trifluoroethylidene)-1,3-dithianes. New intermediates for the preparation of trifluoromethyl-containing compounds
By Solberg, Jan; Benneche, Tore; Undheim, Kjell
From Acta Chemica Scandinavica (1989), 43(1), 69-73. Language: English, Database: CAPLUS, DOI:10.3891/acta.chem.scand.43-0069
An efficient synthesis of the title compds. I (R = H, Me) is described. Oxidn., redn., and acetylation reactions of I are also carried out.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
307. Electrochemical trifluoromethylation of carbonyl compounds
By Sibille, S.; Mcharek, S.; Perichon, J.
From Tetrahedron (1989), 45(5), 1423-8. Language: English, Database: CAPLUS, DOI:10.1016/0040-4020(89)80140- 1
The electroredn. of CF3Br in DMF contg. aldehydes or ketones, using a sacrificial Zn anode, affords the corresponding trifluoromethyl alcs. together with the unreactive organo-Zn species CF3ZnBr and (CF3)2Zn. The alcs. are obtained with good yields from the aldehydes. With the ketones, the organo-Zn species are formed preferentially to the alcs., but the addn. of tetramethylethylenediamine allows the alcs. to be formed in moderate yields.
~27 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
308. Facile synthesis of trifluoroand hexafluoroisopropyl halides
By Hanack, Michael; Ullmann, Joerg
From Journal of Organic Chemistry (1989), 54(6), 1432-5. Language: English, Database: CAPLUS, DOI:10.1021/jo00267a036
Triand hexafluoroisopropyl nonaflates reacted with halide-anions allowing an easy approach to the synthetically interesting fluorinated iso-Pr halides. Thus, treating CF3CH(ONf)CR3 (I; R = H, F) with MX (M = Li, Na; X = Cl, Br, I) in acetylacetone at 25-160° for 16-80 h gives CF3CHXCR3 (II, R = H, F; X = Cl, Br, I) in high yield. I were prepd. by reaction of CF3CH(OH)CR3 (R = H, F) with C4F9SO2F contg. NET3. 1H-, 13C-, 19F-NMR, IR and MS data for I and II are given.
~14 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
309. Oxidation of fluoroalkyl-substituted carbinols by the Dess-Martin reagent
By Linderman, Russell J.; Graves, David M.
From Journal of Organic Chemistry (1989), 54(3), 661-8. Language: English, Database: CAPLUS, DOI:10.1021/jo00264a029

SciFinder® |
Page 192 |
The efficient oxidn. of mono-, di-, tri-, and perfluoroalkyl-substituted carbinols has been accomplished by the Dess-Martin periodinane (I) oxidant. A variety of functional groups are compatible with the oxidn. procedure. Monitoring the oxidn. by 19F-NMR indicated that a discreet periodinane intermediate is formed during the course of the reaction. Nonnucleophilic or sterically encumbered α-thiofluoro carbinols were readily oxidized; nucleophilic α-thio-substituted carbinol n- C8H17SCH2CH(OH)CF3 was not. A tert-Bu alc. modified periodinane oxidant was ultimately employed to achieve oxidn. in this example.
~70 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
310. Kinetics, mechanism, and deuterium isotope effect of the reaction of 1,1,1-trifluoro-2,2-bis(4-nitrophenyl)ethane with sodium phenolate in alcohols. Influence of crown ethers and cryptands
By Schroeder, Grzegorz
From Acta Chimica Hungarica (1988), 125(1), 181-91. Language: English, Database: CAPLUS
The reaction of the title compd. (Ar2CHCF3) with NaOPh in various alcs., such as EtOH, PrOH, BuOH, was investigated. Kinetic measurements show that the elimination of HF from Ar2CHCF3 played a crucial role in detg. the formation rate of Ar2C:CF(OPh), in the alcs. examd.; kinetics for the reaction of Ar2C:CF2 with NaOPh are presented. In EtOD, 100% isotope exchange of H for D in Ar2CHCF3 is reported, while in tert-BuOH, such exchange does not occur at all. It is
concluded that the elimination reaction mechanism changes from (E1cB)R in EtOH to (E1cB)I or to E2cycl. in tert-BuOH. The influence of crown ethers and the 222 cryptand on the Ar2CHCF3 reaction rates and deuterium kinetic isotope effects
are also examd.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
311. Preparation and properties of new methyl-(alkoxo)- and methyl-(thiolato)nickel and methyl(alkoxo)- and methyl(thiolato)-palladium complexes. Carbon monoxide and carbon disulfide insertion into the alkoxo-palladium bond
By Kim, Yong Joo; Osakada, Kohtaro; Sugita, Kouji; Yamamoto, Takakazu; Yamamoto, Akio
From Organometallics (1988), 7(10), 2182-8. Language: English, Database: CAPLUS, DOI:10.1021/om00100a016
Reactions of fluorinated alcs. [HOCH(CF3)2, HOCH2CF3, and HOCH(CF3)Ph] or arom. thiols (HSPh and HSC6H4Me-p) with dialkylnickel and -palladium complexes [NiMe2(bpy), NiEt2(bpy) (bpy = 2,2'-bipyridine), NiMe2(dpe), and PdMe2(dpe) [dpe = 1,2-bis(diphenylphosphino)ethane]] give the corresponding monoalkyl complexes with an alkoxy or a thiolato ligand [NiMe(OR)(bpy), NiEt(OR)(bpy), MMe(OR)(dpe), and MMe(SR)(dpe) [M = Ni, Pd; R = CH(CF3)2, CH2CF3, CH(CF3)Ph]. These complexes were characterized by elemental anal. and NMR [1H, 31P{1H}, 19F, and 13C{1H}] spectroscopy. They react with CO at normal pressure to give carboxylic esters in high yields. Treating NiMe(SR1)(dpe) (R1 = Ph, C6H4Me-p) with CO also gives the corresponding carbothioic esters in good yields, while PdMe(SPh)(dpe) is unreactive with CO under similar conditions. 31P{1H} and 13C{1H} NMR spectra of the reaction mixt. of PdMe[OCH(CF3)2](dpe) with an equimolar amt. of 13CO at -60 ° show formation of PdMe[13COOCH(CF3)2](dpe) via insertion of the CO into the Pd-O bond. At -20 °, this complex gives 1,1,1,3,3,3-hexafluoro-2-Pr acetate via reductive elimination. The reaction is accompanied by simultaneous decarbonylation of the alkoxycarbonyl ligand to regenerate PdMe[OCH(CF3)2](dpe). Reaction of PdMe[OCH(CF3)Ph] (dpe) with CS2 gives an isolable complex, PdMe[SCSOCH(CF3)Ph](dpe), formed by insertion of CS2 into the Pd-O bond, while PdMe(SPh)(dpe) is unreactive with CS2.
~32 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
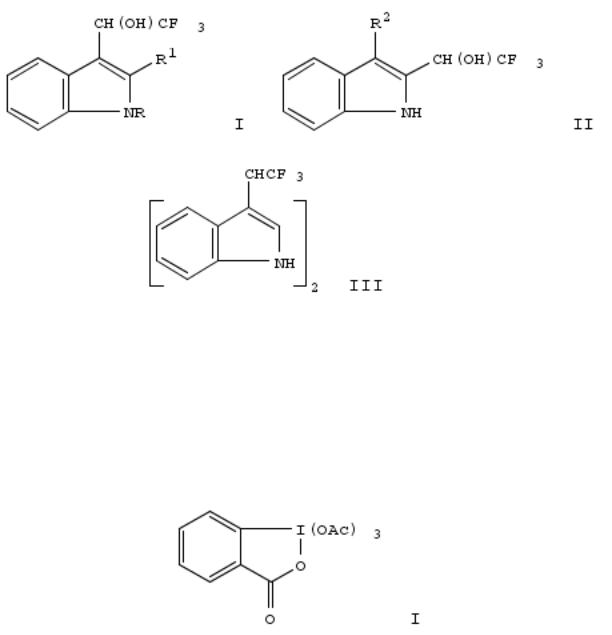
312. Thermal condensation of indoles with trifluoroacetaldehyde
SciFinder® |
Page 193 |
By Maki, Yasuo; Kimoto, Hiroshi; Fujii, Shozo; Senga, Munehiro; Cohen, Louis A.
From Journal of Fluorine Chemistry (1988), 39(1), 47-59. Language: English, Database: CAPLUS, DOI:10.1016/S0022-1139(00)82736-0
The title condensations provide the corresponding (α-hydroxy-β,β,β-trifluoroethyl)indoles. Indole, 1-methylindole, and 2- methylindole gave 3-adducts I (R = H, R1 = H, Me; R = Me, R1 = H) in yields of 77%, 49%, and 84%, resp. 3-Substituted indoles [3-methylindole, Et 3-indoleacetate and 3-(2-acetoxyethyl)indole] afforded 2-adducts II (R2 = Me, CH2CO2Et, CH2CH2OAc) in yields of 69%, 21%, and 23%, I (R = R4 = H) eliminates H2O at 137° to form a transient 3- [(trifluoromethyl)methylene]indolenine, which reacts rapidly with nucleophiles, e.g., indole, to give , e.g., diindolyltrifluoroethane III. I (R = R1 = H) and II (R2 = CH2CO2Et) show weak activity as herbicides but no activity as insecticides or fungicides.
~20 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
313. Efficient procedure for the oxidation of fluorinated carbinols
By Linderman, Russell J.; Graves, David M.
From Tetrahedron Letters (1987), 28(37), 4259-62. Language: English, Database: CAPLUS, DOI:10.1016/S0040- 4039(00)96479-7
The Dess-Martin reagent I was applied to the oxidn. of diand trifluorocarbinols to the corresponding ketones. Benzyl, allyl, propargylic, and aliph. trifluoromethyl carbinols were readily oxidized. n-C8H17SCH2CH(OH)CF3 did not give the ketone.
~15 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
314. Solvent effects on the rates of solvolysis of pinacolyl derivatives
By Roberts, Donald D.; Hall, Edward W.
From Journal of Organic Chemistry (1988), 53(11), 2573-9. Language: English, Database: CAPLUS, DOI:10.1021/jo00246a032

SciFinder® |
Page 194 |
The solvolysis rates of RCH(O3SAr)CMe3 (R, Ar = Et, p-BrC6H4; Me2CH, p-BrC6H4; Me3C, p-tolyl) and CF3CH(O3SR)CMe3 (R = p-BrC6H4, CF3) were detd. in solvent mixts. The results were discussed in terms of electrostatic solvation of carbocations.
~7 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
315. Potential asymmetric syntheses via the ene reaction
By Ben Hassine, B.; Gorsane, M.; Pecher, J.; Martin, R. H.
From Bulletin des Societes Chimiques Belges (1987), 96(10), 801-8. Language: French, Database: CAPLUS
1-(9-Anthryl)ethyl crotonates underwent an addn. reaction with cyclohexene to give cyclohexylbutenoic acid esters I (R1 = Me, CF3, CCl3).
~5 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
316. Kinetic resolution of (E)-[(fluoroalkyl)vinyl]carbinol derivatives by asymmetric epoxidation with titanium-tartrate catalysts
By Hanzawa, Yuji; Kawagoe, Keiichi; Ito, Masayuki; Kobayashi, Yoshiro
From Chemical & Pharmaceutical Bulletin (1987), 35(4), 1633-6. Language: English, Database: CAPLUS, DOI:10.1248/cpb.35.1633
Racemic (E)-RCH:CHCH(OH)R1 (I; R = hexyl, pentyl; R1 = CF3, CF2H, CFH2, CF2Et) were kinetically resolved by Sharpless epoxidn. The optical purity of the resolved alcs. was excellent (98% enantiomeric excess) in I (R = pentyl, R1 = CF2H; R = pentyl, R1 = CFH2) and moderate (60% enantiomeric excess) in I (R = hexyl, R1 = CF3).
~9 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
317. Atrolactic synthesis in the evaluation of the efficiency of inducers of asymmetric synthesis
By Ben Hassine, B.; Gorsane, M.; Geerts-Evrard, F.; Pecher, J.; Martin, R. H.; Castelet, D.
From Bulletin des Societes Chimiques Belges (1986), 95(7), 547, 558-66. Language: French, Database: CAPLUS
(S)-Atrolactic acid was prepd. from PhCOCOCl by sequential esterification with (-)(R)-2,2,2-trifluoro-1-(9-anthryl)ethanol, Grignard addn. with MeI, and sapon. (-)-Quinine and (-)-10,11-dihydroquinine gave (R)-atrolactic acid.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
318. Polyfluoroalkyl dibasic acid phosphates, bis(polyfluoroalkyl) monobasic acid phosphates, and their precursors
By Mahmood, Tariq; Shreeve, Jean'ne M.
From Inorganic Chemistry (1986), 25(21), 3830-7. Language: English, Database: CAPLUS, DOI:10.1021/ic00241a025
SciFinder® |
Page 195 |
The new dibasic acid phosphates RfOP(O)(OH)2 [Rf = (CF3)2CH, CF3(CH3)CH] and monobasic acid phosphates (RfO)2P(O)OH [Rf = CF3(CH3)CH, (CF3)2CH, (CF3)2CH3C, CF3(CH3)2C], as well as new routes to CF3CH2OP(O)(OH)2, (CF3CH2O)2P(O)OH, and [H(CF2)4CH2O]2P(O)OH, are reported. When heated in the presence of water at 80-100°, (RfO)2P(O)OH is hydrolyzed to RfOP(O)(OH)2, which is converted to H3PO4 at higher temps. The dibasic acid phosphates RfOP(O)(OH)2 [Rf = (CF3)2CH3C, CF3(CH3)2C] undergo dehydration to form [RfOP(O)(OH)]2O. Addnl., the chloro precursors to these acids have been synthesized, including the (polyfluoroalkyl)dichlorophosphinites, RfOPCl2 [Rf = CF3CH2, CF3(CH3)CH, (CF3)2CH, (CF3)2CH3C, CF3(CH3)2C], and bis(polyfluoroalkyl)chlorophosphonites, (RfO)2PCl [Rf = CF3CH2, (CF3)2CH3C, CF3(CH3)2C]. If N2O4 is used as an oxidant, the former are converted to RfOP(O)Cl2 and the latter to (RfO)2P(O)Cl. Li polyfluoroalkoxides with PCl3 give tris(polyfluoroalkyl) phosphites, (RfO)3P [Rf = CF3(CH3)CH, (CF3)2CH3C, CF3(CH3)2C, CF3CH2, (CF3)2CH], which can be oxidized to (RfO)3PO phosphates with N2O4. In some cases, (RfO)2PCl [R = (CF3)2CH3C, CF3(CH3)2C] gives tetrakis(polyfluoroalkyl)diphosphates, (RfO)2P(O)OP(O)(ORf)2, and CF3CH2OPCl2 gives CF3CH2OP(O)(μ-O)2P(O)OCH2CF3. The (RfO)3P phosphites [Rf = CF3CH2, CF3(CH3)CH, (CF3)2CH3C, CF3(CH3)2C], undergo Arbuzov rearrangements with Cl2 to form (RfO)2P(O)Cl. HCl converts [CF3(CH3)2CO]3P to [CF3(CH3)2CO]2P(O)H, which, with Cl, forms [CF3(CH3)2CO]2P(O)Cl.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
319. Some new highly substituted (trifluoromethyl)sulfuranes
By Gupta, Krishna D.; Shreeve, Jean'ne M.
From Journal of Fluorine Chemistry (1987), 34(3-4), 453-60. Language: English, Database: CAPLUS, DOI:10.1016/S0022-1139(00)85186-6
trans-CF3SF4Cl readily undergoes reductive defluorination to S(IV)-contg. compds. when reacted with N- or O-contg. nucleophiles. Thus, 20-30% CF3S(NR2)2Cl (R2NH = piperidine, 2,6-dimethylpiperidine, 2,2,6,6-tetramethylpiperidine, morpholine, 3,5-dimethylmorpholine, etc.) were obtained by treating CF3SF4Cl with R2NH and Et3N. CF3SF4Cl reacted with R1OH (R1 = CF3CH2, CF3CHMe) to give CF3S(OR1)2Cl. Because of the low stability of all of these compds., complete characterization was difficult.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
320. Synthesis of polyfluoroalkyl esters of (fluorosulfonyl)difluoroacetic acid and diesters of sulfonyldifluoroacetic acid
By Huang, Ting Ji; Dong, Zhi Xia; Shreeve, Jean'ne M.
From Inorganic Chemistry (1987), 26(14), 2304-6. Language: English, Database: CAPLUS, DOI:10.1021/ic00261a027
Several new polyfluoroalkyl(fluorosulfonyl)difluoroacetates, RO2CCF2SO2F [R = CF3CH2, CF3CF2CH2, CF3CF2CH2, (CF3)2CMe, CF3CHMe, (CF3)2CH, C7F15CH2, CH2(CF2)3CH2O2CCF2SO2F], diesters of sulfonyldifluoroacetic acid, RO2CCF2SO2OR1 [R = CF3CH2, (CF3)2CH; R1 = CF3CH2, (CF3)2CH], and alkyl (fluorosulfonyl)difluoroacetates,
RCHn(CH2O2CCF2SO2F)3-n [R = CH3, n = 0; R = O2N, n = 0; R = O2CCF2SO2F, n = 2; R = CH2O2CCF2SO2F, n = 0] resulted from the reaction of tetrafluoroethane-β-sultone with polyfluoroalkyl alcs. or polyfluoroalkoxides and alkanediols,
-triols, and a -tetraol.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
321. Generation and thermal polymerization of 1-fluoro-2-phenylacetylene
By Okano, T.; Ito, K.; Ueda, T.; Muramatsu, H.
From Journal of Fluorine Chemistry (1986), 32(4), 377-88. Language: English, Database: CAPLUS, DOI:10.1016/S0022-1139(00)81946-6
The synthesis of PhC≡CF (I was attempted. The halogen exchange of chloroand bromophenylacetylenes and dehydrobromination of PhCHBrCHFBr were unsuccessful. Defluorosilylation of Me3SiPhC:CF2, (which was prepd. by lithiation of PhCBr:CF2 followed by silylation) with CsF gave an oligomer of I. The reaction of II with CsF in DMF in the presence of PhN3 afforded a 1,3-dipolar cycloadduct, 5-fluoro-1,4-diphenyl-1,2,3-triazole. Vapor phase vacuum pyrolysis of the II yielded I, which spontaneously polymd.
~15 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 196 |
322. Asymmetric syntheses and potential asymmetric synthesis of a-amino alcohols: hydroxyamination of olefins by the sharpless method
By Ben Hassine, B.; Gorsane, M.; Pecher, J.; Martin, R. H.
From Bulletin des Societes Chimiques Belges (1985), 94(11-12), 759-69. Language: French, Database: CAPLUS
Optically active a-amino alcs. were synthesized by the Sharpless method using (-)-10,11-dihydroquinine (I) and (R)-(-)- pantolactone as chiral inducers (R1OH). (dl)-2-Hydroxyheptahelicene (II) and 5 secondary (dl) alcs. were also used to prep. the intermediate diastereomeric (dl)a-hydroxy carbamates III. The highest inductions (e.e. ³98%) were obtained with (E)-stilbene and I or II.
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
323. Fluoro-containing methacrylic acid esters for contact lenses
By Yokoyama, Kazumasa; Yamauchi, Koichi; Inoue, Toshihisa; Osawa, Nobuyuki; Kameda, Nobuo; Oosawa, Nobuyuki
From Jpn. Kokai Tokkyo Koho (1986), JP 61109756 A 19860528, Language: Japanese, Database: CAPLUS
A F-contg. methacrylate CH2CMeCO2CHR(CF2)nCF3 (R = Ph, pentafluorophenyl, cyclohexyl; n = 0-8) is useful in manuf. of an O-permeable fluoropolymer for a hard contact lens. Thus, 100 g decafluorobenzhydrol in 1L benzene was treated with 450 g methacryloyl chloride in the presence of 300 g Et3N at room temp. in 8 h and refluxed for 15 h to give 105 g 1,1-bis(pentafluorophenyl) Me methacrylate (I). A copolymer from I 2.0, 2-hydroxyethyl methacrylate 0.2, 3- (methacryloyloxy)propylbis(trimethylsiloxy) methylsilane 1.0, Me methacrylate 1.5, and ethylene glycol dimethacrylate 0.2 g exhibited O permeation rate 12.0 × 10-11 cm3.cm/cm2.s.mm Hg and Vickers hardness 95.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
324. Thermal condensation of imidazole with trifluoroacetaldehyde
By Fujii, Shozo; Maki, Yasuo; Kimoto, Hiroshi; Cohen, Louis A.
From Journal of Fluorine Chemistry (1986), 30(4), 415-28. Language: English, Database: CAPLUS, DOI:10.1016/S0022-1139(00)85096-4
The condensation of imidazole with CF3CH(OH)OMe occurred readily at reflux to give 4-(1-hydroxy-2,2,2- trifluoroethyl)imidazole (37.3%), 2-(1-hydroxy-2,2,2-trifluoroethyl)imidazole (8.8%), 2,4-bis(1-hydroxy-2,2,2- trifluoroethyl)imidazole (7.2%), and 4,5-bis-product (0.4%). (Trifluoroacetyl)imidazoles were prepd. by oxidn. of these condensation products. Nitration and bromination of the condensation products gave the corresponding nitroand bromoimidazoles, resp.
~5 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
325. The acetal group. I. Acetals of (halomethyl)arylcarbinols
By Knollmueller, Max; Noe, Christian R.; Oberhauser, Berndt
From Monatshefte fuer Chemie (1986), 117(3), 407-19. Language: German, Database: CAPLUS
Diastereomeric acetals I (R = R1R2CH; R1 = Me, ClCH2, Cl2CH, CF3; R2 = Ph, 1-naphthyl, 9-anthryl) of methanobenzofuranol were prepd. by reactions of I (R = H) with (±)-R1R2CHOH [(±)-II]. The diastereomers were sepd. by column chromatog. Methanolysis of these I gave (S)- and (R)-II and I (R = OMe).

SciFinder® |
Page 197 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
326. Absolute configuration of secondary alcohols determined by gas chromatography
By Schoenauer, Kurt J.; Walter, Peter; Noe, Christian R.
From Monatshefte fuer Chemie (1986), 117(1), 127-30. Language: English, Database: CAPLUS, DOI:10.1007/BF00809180
The abs. configuration of secondary alcs., e.g., PhCHMeOH, was detd. by the characteristic gas chromatog. peaks of their acetals obtained from their reaction with (+)-MBF-OH (I) due to the enantiomeric selectivity in acetalization.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
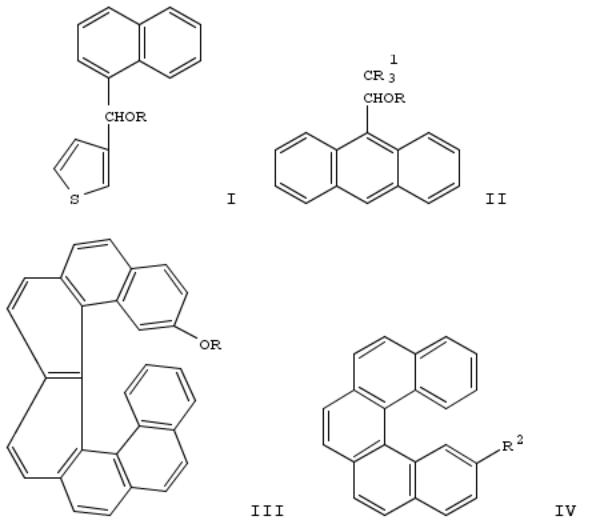
327. Diastereoselective sodium borohydride reductions of (dl)-α-keto esters
By Ben Hassine, B.; Gorsane, M.; Pecher, J.; Martin, R. H.
From Bulletin des Societes Chimiques Belges (1985), 94(8), 597-603. Language: English, Database: CAPLUS
The (±)-alcs. of naphthalene I (R = H), anthracenes II (R = H, R1 = Cl, Br, F), and heptahelicene III (R = H) were esterified with PhCOCOCl to give the (±)-esters I-III (R = COCOPh), which were reduced with NaBH4 in 99:1 THF-MeOH to give 80-99% (±)-esters I-III (R = COCHPhOH) with 54 to 100% diastereomeric excesses. III (R = H) was prepd. from pentahelicene IV (R2 = CO2Me) by redn. and oxidn. to IV (R2 = CHO), coupling with 4-MeOC6H4CH2P+Ph3 Br-, cyclization to III (R = Me), and demethylation.
SciFinder® |
Page 198 |
~12 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
328. Deamination reactions. 43. The effect of trifluoromethyl groups on the reactivity of aliphatic diazonium ions and carbocations
By Gassen, Karl Rudolf; Kirmse, Wolfgang
From Chemische Berichte (1986), 119(7), 2233-48. Language: German, Database: CAPLUS
Various trifluoroalkanamines [H2NCHEtCF3, H2NCHMeCH2CF3, CF3(CH2)3NH2, etc.] were prepd. and diazotized (water, pH 3.5) to probe the effect of trifluoromethyl groups on the reactivity of aliph. diazonium ions. The product distributions reveal that α-CF3 groups enhance inverting displacement and enforce rearrangements (hydride shifts) sepg. the pos. charge from CF3. Migrations of the pos. charge from the β- to the γ-position are less strongly promoted than those from α to β. Enhancement factors of ca. 15 (α→β) and 4 (β→γ) may be derived by comparison with analogous alkanediazonium ions. The pos. charge does not migrate in the reverse direction (β→α) except for minor amts. of a pinacolic rearrangement CF3CMe(OH)CH2NH2 → CF3COEt. A migration of the pos. charge from γ to β has been detected with CF3(CH2)3N2 + but a ten-fold decrease as compared to the analogous butanediazonium ion is indicated. All observations are reasonably explained in terms of the relative stabilities of the intermediate trifluoroalkyl cations.
~7 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
329. Solvolysis of 1-(1-naphthyl)- and 1-(9-anthryl)-2,2,2-trifluoroethyl sulfonates
By Allen, Annette D.; Girdhar, Rabindra; Jansen, Michael P.; Mayo, James D.; Tidwell, Thomas T. From Journal of Organic Chemistry (1986), 51(8), 1324-9. Language: English, Database: CAPLUS, DOI:10.1021/jo00358a031

SciFinder® |
Page 199 |
The m values for the solvent effects on the solvolysis of R1CH(O3SR)CF3 [I; R = 1-naphthyl, R = p-tolyl (II); R1 = 9- anthryl, R = Me (III)] were detd.; the reactivity of I decreases in the order III > II > I (R1 = p-tolyl). The substitution products from III in EtOH, HOAc, or CF3CH2OH involve extensive or exclusive ring attack. A comparison of polarimetric and product rate consts. of (R)-(-)-III suggests that I react by initially forming intimate ion pairs, which either return to reactant or form products through further steps which may involve other ion pairs. Concurrent solvent attack and sulfonate departure is not obsd. The ability of the α-CF3 group in III to enhance solvolytic ring attack is discussed.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
330. The synthesis of α,α-difluoroaldehydes and ketones via Claisen rearrangements
By Metcalf, Brian W.; Jarvi, Esa T.; Burkhart, Joseph P.
From Tetrahedron Letters (1985), 26(24), 2861-4. Language: English, Database: CAPLUS, DOI:10.1016/S0040- 4039(00)98856-7
Claisen rearrangements of reactants contg. 2 F atoms in either the allyl or vinyl fragment were described. E.g., Claisen rearrangement of F2C:CROCH2CH:CHPh (R = H, Me3Si), prepd. from CF3CH2OCH2CH:CHPh, gave RCOCF2CH(CH:CH2)Ph. Similarly, orthoacetate Claisen rearrangement of PhCH2CH(OH)C(OTs):CF2 (Ts = tosylate) gave PhCH2CH:C(OTs)CF2CH2CO2Me as a 94:6 mixt. of isomers. Also prepd. by an Ireland enolate Claisen reaction was PhCH2CH:C(OPh)CF2CHMeCO2H.
~32 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
331. Syntheses and reactions of polyfluoroalkyl fluorosulfates
By Huang, Tingji; Shreeve, Jean'ne M.
From Inorganic Chemistry (1986), 25(4), 496-8. Language: English, Database: CAPLUS, DOI:10.1021/ic00224a020
Polyfluoroalkyl fluorosulfates, ROSO2F [R = F3CCHMe, F3CCMe2 (F3C)2CH] were prepd. by reaction of polyfluoro alcs. with sulfuryl fluoride or sulfuryl chloride fluoride. They reacted with nucleophilic reagents such as amines, polyfluoro alcs., polyfluoroalkoxides and bromide ion to give sulfamates, dialkyl sulfates, and polyfluoroalkyl bromides, resp. In the case of F3CCMe2O3SF with bromide ion, the polyfluoroalkyl bromide loses HBr to give F3CCMe:CH2 in high yield.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
332. Addition of nucleophilic reagents to isopropyland hydroxyisopropyl(trifluoromethyl)diacetylenes
By Turbanova, E. S.; Orlova, N. A.; Stepanova, N. P.; Petrov, A. A.
From Zhurnal Organicheskoi Khimii (1985), 21(5), 974-9. Language: Russian, Database: CAPLUS
Addn. reaction of MeOH to CF3C≡CC≡CCRMe2 (I; R = H) in the presence of NaOMe at 55-60° gave CF3CX:CHC≡CCRMe2 (II; X = OMe, R = H), which was hydrogenated over Pd/CaCO3 to give CF3CH(OMe)(CH2)3CHMe2 (III). III was also prepd. by treating CF3CHO successively with Me2CH(CH2)3MgBr, Na and Me2SO4. Cadiot-Chodkiewicz reaction of CF3C≡CBr with HC≡CCMe2OH gave 51.2% I (R = OH), which reacted at room temp. with MeOH and -20 to -25° with Me2NH and with EtSH-KOH to give 60.5-75% II (X = OMe, NMe2, SEt, resp.; R = OH). Analogous reaction with N2H4.H2O at 100-110° gave 80% pyrazole IV but no CF3C(:NNH2)CH2C≡CCMe2OH.
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 200 |
333. Asymmetric electrophilic substitution on phenols. Enantioselective ortho-hydroxyalkylation mediated by chiral alkoxyaluminum chlorides
By Bigi, Franca; Casiraghi, Giovanni; Casnati, Giuseppe; Sartori, Giovanni; Gasparri Fava, Giovanna; Ferrari Belicchi, Marisa
From Journal of Organic Chemistry (1985), 50(25), 5018-22. Language: English, Database: CAPLUS, DOI:10.1021/jo00225a003
Ortho-specific hydroxyalkylation of phenols with chloral in the presence of chirally modified aluminum chloride derivs. gave enantiomeric excesses of up to 80%, using (-)-menthoxy(ethyl)aluminum chloride in PhMe at room temp. A chelate transition state involving the chiral Lewis acid promoter and the reactants is proposed to account for both regioand enantioselection.
~44 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
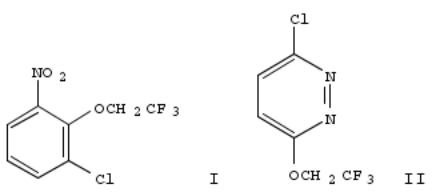
334. Regioselective fluoroalkoxylation and polyfluoroalkoxylation of aromatic and heteroaromatic polyhalides
By Gupton, John T.; Hertel, George; DeCrescenzo, Gary; Colon, Cesar; Baran, Donna; Dukesherer, Dan; Novick, Steve; Liotta, Dennis; Idoux, John P.
From Canadian Journal of Chemistry (1985), 63(11), 3037-42. Language: English, Database: CAPLUS, DOI:10.1139/v85-504
A series of activated polyhalobenzenes and polyhaloheteroarom. compds. were treated with a variety of fluoroalkoxide anions. In most cases, regioselective monosubstitution or polysubstitution was obsd. E.g., 1 mol CF3CH2ONa gave I with 2,3-Cl2C6H3NO2 and II with 3,6--dichloropyridazine.
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
335. New stereoselective approach to acryl esters and its application in sugar chemistry
By Vatele, Jean Michel
From Carbohydrate Research (1985), 136, 177-85. Language: French, Database: CAPLUS
Regioand stereoselective synthesis of an acryl residue by treatment of an allyl alc. with (Z,E)-1-fluoro-1-methoxy-2- (phenylsulfinyl)propene was performed in 3 consecutive reactions in the same flask; displacement of F- by the alc., Claisen rearrangement, and dehydrosulfinylation. Thus, 1,5-anhydro-2-deoxy-4,6-O-isopropylidene-D-arabino-hex-1- enitol gave Me 3,7-anhydro-6,8-O-isopropylidene-2,4,5-trideoxy-2-C-methylene-D-ribo-oct-4-enonate and Me 2-O-allyl- 4,6-dideoxy-α-D-erythro-hex-4-enopyranoside gave Me (Me 2-O-allyl-3,4,6-trideoxy-5-C-methyl-6-C-methylene-α-L- threo-hept-3-enopyranosid)uronate, which has a chiral quaternary C-5 atom.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
336. Catalytic phosphorylation of polyfluoroalkanols. 10. Catalytic phosphorylation of α-(polyfluoroalkyl)benzyl alcohols with diaryl chlorophosphates
By Goryunov, E. I.; Zakharov, L. S.; Petrovskii, P. V.; Kabachnik, M. I.
From Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya (1985), (4), 878-82. Language: Russian, Database: CAPLUS
