
Reference_01_08_2014_165529
.pdf
SciFinder® |
Page 131 |
The invention relates to substituted 3-amino-pyrrolidine-4-lactam derivs. I, processes for prepg. them, pharmaceutical prepns. comprising them, and their pharmaceutical use. I are inhibitors of dipeptidyl peptidase IV (DPP-IV), useful in the treatment of, e.g., diabetes type 2. In compds. I, A is (CH2)n, wherein n is 1 or 2; R1 and R2 are independently H or F; R3 is (un)substituted heteroaryl, etc.; including pharmaceutically acceptable salts thereof. For instance, the invention compd. II was prepd. and showed dipeptidyl peptidase inhibition IC50 value of 24.1 nM.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
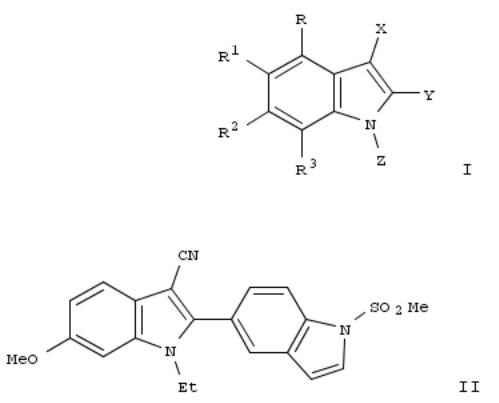
183. Preparation of substituted indoles for treating hepatitis C
By Karp, Gary M.; Hwang, Peter Seongwoo; Takasugi, James J.; Ren, Hongyu; Wilde, Richard Gerald; Turpoff, Anthony A.; Arefolov, Alexander; Chen, Guangming; Campbell, Jeffrey A.
From U.S. Pat. Appl. Publ. (2007), US 20070299068 A1 20071227, Language: English, Database: CAPLUS
The title compds. I [X = NO2, CO2H, halo, etc.; Y = (un)substituted benzothiazolyl, indolyl, etc.; Z = alkyl optionally substituted with 5-6 membered heterocyclyl, or 5-6 membered heterocyclyl; R = H; R1 = H, 5-6 membered heterocyclyl, (un)substituted alkyl, etc.; R2 = (un)substituted alkyl, alkylthio, alkyl, etc.; R3 = H; with the provisos], useful for treating Hepatitis C viral infection, were prepd. Thus, treating 1-ethyl-6-methoxy-1H,1'H-[2,5']biindolyl-3-carbonitrile with methanesulfonyl chloride afforded 81% II which showed IC50 between 0.5 μM and 2 μM when tested in HCV replicon or HCV-PV systems. This invention provides also pharmaceutical compns. comprising compds. I, and methods of using such compds. or compns. for treating infection by a virus, or for affecting viral IRES activity.
SciFinder® |
Page 132 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
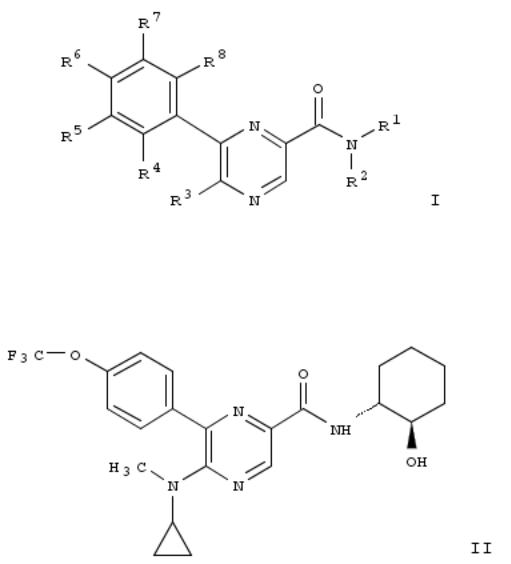
184. Preparation of pyrazinecarboxamide derivatives as CB1 antagonists
By Hebeisen, Paul; Iding, Hans; Nettekoven, Matthias Heinrich; Sander, Ulrike Obst; Roever, Stephan; Weiss, Urs; Wirz, Beat
From U.S. Pat. Appl. Publ. (2007), US 20070293509 A1 20071220, Language: English, Database: CAPLUS
Title compds. I [R1 = cycloalkyl (optionally substituted with hydroxy, alkoxy or hydroxyalkyl), -CH2-(CR9R10)m-cycloalkyl, piperidinyl, etc.; R9 = H, alkyl or cycloalkyl; R10 = H, hydroxy or alkoxy; m = 0, 1; R2 = H; R3 = -OR14; R14 = alkyl, haloalkyl, cycloalkyl, etc.; R4 = H or halo; R5 = H, halo, haloalkyl, etc.; R6 = H, halo, haloalkyl, etc.; R7 = H, halo, haloalkyl, etc.; R8 = H or halo] and their pharmaceutically acceptable salts were prepd. Thus, a multi-step synthesis of compd. II, starting from 3,5-dibromopyrazin-2-ylamine, was given. In CB1 receptor (cannabinoid receptor, type CB1) affinity assays, the Ki value of compd. II was 0.0354 μM. Compds. I are claimed useful for the treatment of an eating disorder, obesity or type II diabetes. Pharamceutical compn. comprising compds. I is disclosed.
SciFinder® |
Page 133 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
185. Tandem enzyme-catalyzed reduction/cis-dihydroxylation of 2,2,2-trifluoroacetophenone: chemoenzymatic routes to new enantiopure phenol and benzylic alcohol reagents
By Boyd, Derek R.; Sharma, Narain D.; Ljubez, Vera; Malone, John F.; Allen, Christopher C. R.
From Journal of Chemical Technology and Biotechnology (2007), 82(12), 1072-1081. Language: English, Database: CAPLUS, DOI:10.1002/jctb.1686
Factors that control the competition between toluene dioxgenase-catalyzed arene cis-dihydroxylation and dehydrogenase-catalyzed ketone redn. have been studied, using whole cells of Pseudomonas putida UV and three alkylaryl ketones. The triol metabolite, obtained from 2,2,2-trifluoroacetophenone, has been used in the synthesis of single enantiomer chiral phenols and benzylic alcs. Potential applications of the methyl-ether derivs. of the chiral phenols and benzylic alcs., as resolving agents, have been found.
~5 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
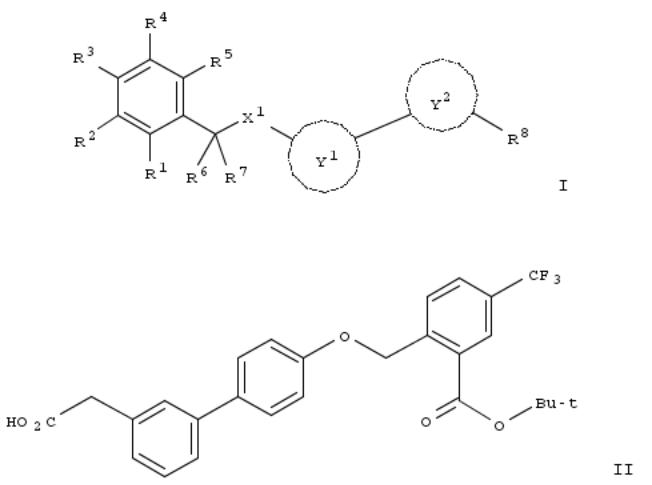
186. Preparation of benzene compounds having two or more substituents as liver X receptors (LXR) modulators
By Tamaki, Kazuhiko; Yamaguchi, Takahiro; Oda, Kozo; Terasaka, Tadao; Nakai, Daisuke; Nakadai, Masakazu From Jpn. Kokai Tokkyo Koho (2007), JP 2007314516 A 20071206, Language: Japanese, Database: CAPLUS
SciFinder® |
Page 134 |
The title compds. [I; R1 = COR9; R9 = C1-10 alkyl, C1-10 (halo)alkoxy, phenyl-C1-10 alkoxy, mono or di(C1-10 alkyl)amino, etc.; R2 = H, halo-C1-4 alkyl, HO, C1-4 alkoxy, NH2, monoor di(C1-4 alkyl)amino, halo; R3 = H, C1-6 (halo)alkyl, C1-4 alkoxy-C1-4 alkyl, C1-4 alkylthio-C1-4 alkyl, C1-4 alkylsulfinyl-C1-4 alkyl, C1-4 alkylsulfonyl-C1-4 alkyl, N-mono- or di(C1-4 alkyl)amino-C1-4 alkyl, C3-6 cycloalkyl, C2-6 alkenyl, HO, C1-6 alkoxy, etc.; R4, R5 = H, C1-4 alkyl, halo-C1-4 alkyl, C3-6 cycloalkyl, HO, C1-4 (halo)alkoxy, halo; R6, R7 = H, C1-3 alkyl; R8 = -X2R10; R10 = COR11, SO2R12, NR13COR14, tetrazol- 5-yl, etc.; R11 = C1-6 alkyl, HO, C1-6 alkoxy, C3-8 cycloalkyl-C1-6 alkyloxy, C3-8 cycloalkyloxy, (un)substituted NH2, etc.; R12 = C1-6 alkyl, C3-8 cycloalkyl-C1-6 alkyl, C3-8 cycloalkyl, (un)substituted NH2, etc.; R13, R14 = H, C1-6 alkyl, C3-8 cycloalkyl- C1-6 alkyl, C3-8 cycloalkyl; X2 = single bond, (un)substituted C1-4 alkylene; X1 = NH, C1-4 alkyl-NH, O, S, SO, SO2; Y1 = each (un)substituted Ph or 5- o 6-membered ring heterocyclyl; Y2 = each (un)substituted 6- to 10-membered ring aryl, 9- or 10-membered ring unsatd. cyclic hydrocarbyl, 5- to 10-membered ring arom. heterocyclyl, or 5- to 10-membered ring unsatd. heterocyclyl] or pharmaceutically acceptable salts or esters thereof are prepd. These compds. are modulators or agonists of liver X receptors (LXR) and are useful for the prophylaxis and/or treatment of arteriosclerosis, atherosclerosis, diabetes-induced arteriosclerosis, hyperlipidemia, hypercholesteremia, lipid-related diseases, inflammatory diseases, arteriosclerotic heart diseases, cardiovascular diseases, coronary artery disease, or diabetes. Thus, Suzuki coupling of tert-Bu 2-[[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy]methyl]-5-(trifluoromethyl)benzoate with 3- bromophenylacetic acid in the presence of tetrakis(triphenylphosphine)palladium(0) and K2CO3 in aq. DMF at 110° for 6
h gave (4'-[[2-(tert-butoxycarbonyl)-4-(trifluoromethyl)benzyl]oxy]-1,1'-biphenyl-3-yl)acetic acid (II). II showed EC50 of ≤5 and ≤3 μM for activating transcription of human LXRα and human LXRβ, resp., in African green monkey (CV-1) cells expressing human LXRα and LXRβ. Pharmaceutical formulations, e.g. a hard capsule contg. [4'-[[2-(tert- Butoxycarbonyl)-4-fluoro-3-hydroxybenzyl]oxy]-1,1'-biphenyl-4-yl]acetic acid, were also prepd.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
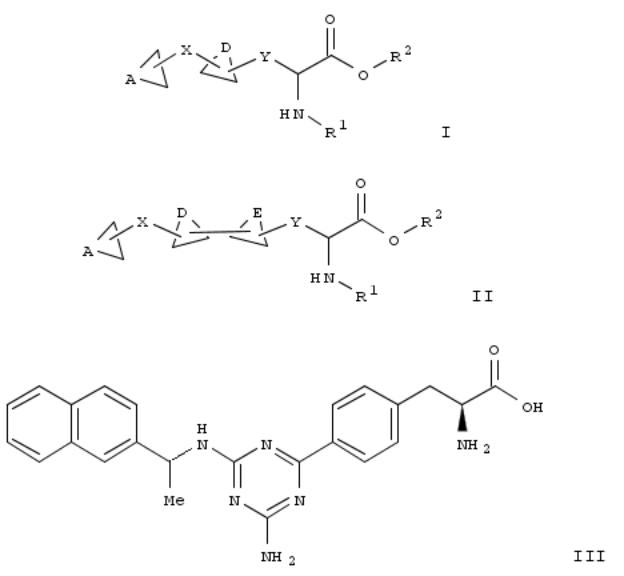
187. Preparation of multicyclic amino acid derivatives and their use as TPH1 inhibitors for treating serotonin-mediated diseases
By Devasagayaraj, Arokiasamy; Jin, Haihong; Liu, Qingyun; Marinelli, Brent; Samala, Lakshama; Shi, Zhi-Cai; Tunoori, Ashok; Wang, Ying; Wu, Wenxue; Zhang, Chengmin; et al
From PCT Int. Appl. (2007), WO 2007089335 A2 20070809, Language: English, Database: CAPLUS
SciFinder® |
Page 135 |
Title compds. I and II [A = (un)substituted cycloalkyl, (hetero)aryl; X = a bond, S, O, CO, CH and derivs., C≡C, SO2, etc.; D, E = independently (un)substituted (hetero)aryl; R1, R2 = independently H, (un)substituted alkyl, alkylaryl, alkylheterocyclyl, aryl, heterocyclyl; Y = (CH2)n; n = 0-3; and their pharmaceutically acceptable salts and solvates] were prepd. as tryptophan hydroxylase isoform 1 (TPH1) for treating, preventing and managing serotonin-mediated diseases and disorders (no data). Thus, thus amination of 2-amino-4,6-dichloro-1,3,5-triazine with (R)-(+)-1-(2- naphthyl)ethylamine, and coupling of the chloride (no data) with 4-borono-L-phenylalanine gave 4-substituted phenylalanine III. Potent TPH1 inhibitors I and II reduced 5-hydroxytryptamine levels in both the small and large intestine, but not in the brain of mice following their oral administration. I and II are useful for treating carcinoid syndrome, emesis, diarrhea, constipation, and irritable bowel syndrome (no data).
~15 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
188. Preparation of 3-chloro-4-isopropoxybenzamide and 3-cyano-4-isopropoxybenzamide derivatives as inhibitors of mitotic kinesins
By Qian, Xiangping; Ashcraft, Luke W.; Wang, Jianchao; Yao, Bing; Jiang, Hong; Bergnes, Gustave; Morgan, Bradley P.; Morgans, David J.; Dhanak, Dashyant; Knight, Steven D.; et al
From U.S. Pat. Appl. Publ. (2007), US 20070149516 A1 20070628, Language: English, Database: CAPLUS

SciFinder® |
Page 136 |
The title compds. [I; R1 = 3-halo-4-((R)-1,1,1-trifluoropropan-2-yloxy)phenyl, 3-cyano-4-((R)-1,1,1-trifluoropropan-2- yloxy)phenyl, 3-halo-4-isopropylaminophenyl, 3-cyano-4-isopropylaminophenyl, 3-halo-4-((R)-1,1,1-trifluoropropan-2- ylamino)phenyl, 3-cyano-4-((R)-1,1,1-trifluoropropan-2-ylamino)phenyl; X = CO, SO2; R2 = H, (un)substituted lower alkyl; W = CR4, CH2CR4, N; R3 = COR7, H, each (un)substituted substituted alkyl, heterocycloalkyl, heteroaryl, or aryl, cyano, sulfonyl; R4 = H, (un)substituted alkyl; R5 = H, HO, each (un)substituted amino, cycloalkyl, heterocycloalkyl, heteroaryl, or lower alkyl; R6 = H, CONH2, (un)substituted alkyl, alkoxy, aryloxy, heteroaryloxy, alkoxycarbonyl, aryl, heteroaryl, cycloalkyl, or heterocycloalkyl; R7 = HO, each (un)substituted lower alkyl, aryl, amino, aralkoxy, or alkoxy; provided that if W is N, then R5 is not hydroxy or (un)substituted amino, and R6 is not optionally substituted alkoxy, optionally substituted aralkoxy, optionally substituted heteroaralkoxy, or optionally substituted amino] are prepd. (1R)-1- (methoxycarbonylamino)-1-[4-[4-[(2S)-2-[[[4-(((1R)-2,2,2-trifluoroisopropyl)oxy)-3-chlorophenyl]carbonyl]amino]-4- hydroxybutyl]phenyl]-1-ethylimidazol-2-yl]ethane. These compds. including N-benzoyl-amino alcs., N-benzoyl-amino acid amide, N-benzoylsemicarbazide, and N-benzoyl-diamine derivs. are inhibitors of one or more mitotic kinesins and are useful in the treatment of cellular proliferative diseases, for example cancer, hyperplasias, restenosis, cardiac hypertrophy, immune disorders, fungal disorders, and inflammation by modulating the activity of one or more mitotic kinesins. Thus, cyclocondensation of (2S)-2-(tert-butoxycarbonylamino)-5-bromo-4-oxopentanoic acid Me ester with thiobenzamide in the presence of diisopropylethylamine in methanol under refluxing for 24 h gave (2S)-2-(tert- butoxycarbonylamino)-3-(2-phenylthiazol-4-yl)propanoic acid which was treated with CF3CO2H in CH2Cl2 at room temp. for 10 min to give (2S)-2-amino-3-(2-phenylthiazol-4-yl)propanoic acid (II). II was condensed with 3-chloro-4- isopropoxybenzoic acid pentafluorophenyl ester in the presence of diisopropylethylamine in DMF at room temp. to give (2S)-N-methyl-2-[(3-chloro-4-isopropoxybenzoyl)amino]-3-(2-phenylthiazol-4-yl)propanamide (III). Many of the compds. I showed GI50 (50% growth inhibition concn.) of ≤10 μM against human ovarian tumor cells Skov-3.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
189. Preparation of imidazole derivatives for treatment of cellular proliferative diseases
By Qian, Xianping; Bergnes, Gustave
From PCT Int. Appl. (2007), WO 2007056056 A2 20070518, Language: English, Database: CAPLUS
The title imidazole derivs. with general formula of R1-X-N(R2)-W(R3)-CR4-6 [wherein R1 = (un)substituted (hetero)cycloalkyl or (hetero)aryl; X = CO or SO2; R2 = H or (un)substituted alkyl; W = CR8, CH2CR8, or N; R3 = H, (un)substituted (cyclo)alkyl, heterocycloalkyl, (hetero)aryl, etc.; R4 = halo, (un)substituted alkyl, alkenyl, alkynyl, alkoxy, etc.; R5 = halo, OH, (un)substituted amino, (cyclo)alkyl, etc.; R6 = (un)substituted alkyl, alkenyl, alkynyl, alkoxy, etc.; R8 = H or (un)substituted alkyl; or R4 and R5 form an oxo group; or R4 and R8 form an C=C group wherein R5 is chosen from H or (un)substituted alkyl], or pharmaceutically acceptable salts, solvates, chelates, complexes, prodrugs, or mixts. thereof were prepd. for the treatment of cellular proliferative diseases, such as cancer, hyperplasias, restenosis, etc. For example, I was prepd. in a multi-step synthesis. Test of the inhibitory activity of the title compds. against human ovarian tumor cells was described (no data).

SciFinder® |
Page 137 |
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
190. Preparation of chromone-substituted 1,4-dihydropyridines as antagonists of the mineralocorticoid receptor (MR) for treating cardiovascular diseases
By Kuhl, Alexander; Kolkhof, Peter; Heckroth, Heike; Schlemmer, Karl-Heinz; Flamme, Ingo; Figueroa Perez, Santiago; Gielen-Haertwig, Heike; Grosser, Rolf; Ergueden, Jens-Kerim; Lang, Dieter
From Ger. Offen. (2007), DE 102005034267 A1 20070125, Language: German, Database: CAPLUS
Title compds. [I; R1, R2 = alkyl, CF3, cyclopropyl, cyclobutyl; R3 = cycloalkyl, (substituted) alkyl, Ph; R4 = (substituted) alkyl, cycloalkyl; R5 = H, halo, cyano, NO2, CF3, alkyl, alkoxy; R6 = H, F] and salts and solvates thereof, were prepd. Thus, a mixt. of 2-methyl-4-oxo-4H-chromene-8-carboxaldehyde (prepn. given), Pr 3-oxobutanoate, 3-amino-1-(4- fluorophenyl)but-2-en-1-one (prepn. given), and MeCO2H in 2-propanol was heated under reflux for 30 h to give 87% I (R1, R2 = Me; R3 = 4-fluorobenzyl; R4 = propyl; R5, R6 = H). Tested I showed MR antagonist activity with IC50 = 18-235 nM.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
191. Di-2,2,2-trifluoro-1-(9-anthryl)ethyl fumarate, an easy starting point for the enantioselective preparation of trans- cyclohexene-4,5-dicarboxylate derivatives by Diels-Alder reaction

SciFinder® |
Page 138 |
By Palomino-Schaetzlein, Martina; Virgili, Albert; Jaime, Carlos; Molins, Elies
From Tetrahedron: Asymmetry (2007), 17(23), 3237-3243. Language: English, Database: CAPLUS, DOI:10.1016/j.tetasy.2006.11.047
Bis((R)-2,2,2-trifluoro-1-(9-anthryl)ethyl) fumarate was synthesized from fumaric acid and enantiopure Pirkle alc. The Diels-Alder reaction with different dienes employing different reaction conditions was assayed, with high diastereomeric excesses obtained. The structure and geometry of the cycloadducts was analyzed by NMR, mol. mechanics and x-ray diffraction. Hydrolysis made it possible to obtain the enantioenriched trans-cyclohexene-4,5-dicarboxylate derivs. and allowed the authors to recover the chiral auxiliary. The crystal and mol. structures of bis[(R)-1-(9-anthryl)-2,2,2- trifluoroethyl] (1S,2S)-4,5-dimethylcyclohex-4-ene-1,2-dicarboxylate were detd. by x-ray crystallog.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
192. α-Fluorinated cyclic amidophosphite ligands. Their synthesis, Rh complexes and catalytic activity in the hydroformylation of styrene
By Artyushin, Oleg; Odinets, Irina; Goryunov, Evgenii; Fedyanin, Ivan; Lyssenko, Konstantin; Mastryukova, Tatyana; Roeschenthaler, Gerd-Volker; Kegl, Tamas; Keglevich, Gyoergy; Kollar, Laszlo
From Journal of Organometallic Chemistry (2006), 691(26), 5547-5559. Language: English, Database: CAPLUS, DOI:10.1016/j.jorganchem.2006.08.098
The synthetic approaches to cyclic phosphite and amidophosphite ligands bearing electron withdrawing perfluorinated groups at the β-position to the P atom were elaborated. Two general methodologies can be used to obtain these cyclic phosphites. The more common route is based on the so called 'chloride methodol.', wherein the corresponding preformed cyclic P(III) acid chloride interacts with the corresponding alc. in the presence of a base. Benzannulated cyclic phosphites I (Y = O, R = C6H13 (3a), P(O)(OEt)2 (3b)) were obtained by reaction of 2-chloro-1,3,2-benzodioxaphosphole with the corresponding alc. in the presence of NEt3. 1,3,2-Oxazaphospholidines II (Y = O, X = F, R = 3-CF3C6H4 (8a), P(O)(OEt)2 (8b); X = H, R = C6F5 (8c)) and 1,3,2-diazaphospholidines II (Y = NMe, X = F, R = Ph (9a), P(O)(OEt)2 (9b), C6H13 (9c)) were obtained by reaction of 2-chloro-3-methyl-1,3,2-oxazaphospholidine or 2-chloro-1,3-dimethyl-1,3,2- diazaphospholidine, resp. with the corresponding alc. in the presence of NEt3. The treatment of dichlorophosphites by bisnucleophiles is a variant of this method and has been used in the prepn. of fluorinated cyclic diamidophosphites. The mol. structure of one of the cyclic diamidophosphites was detd. by x-ray crystallog. The other route, the so-called 'amide procedure', is based on the interaction of P(III) acid amides with the desired alcs. Benzannulated amidophosphites I (Y =
NMe, X = F, R = Ph (5a), 3-MeOC6H4 (5b), 3-CF3C6H4 (5c), C6H13 (5d); X = H, R = C6F5 (5e)) were obtained in high yields and purities by the 'amide procedure'. Catalytic systems based on Rh complexes of these ligands formed in situ
using Rh(CO)2(acac) (acac = acetylacetonate) as a catalytic precursor demonstrate high activity in the hydroformylation of styrene along with good selectivity in respect to branched aldehyde. Quantum-chem. calcns. proved that both the rate of the formation of branched alkyl complex, as well as its reactivity are influenced by the steric and electronic parameters in the same manner. The monoand the diamidophosphites obtained are suitable P-ligands to give stable Rh(III) complexes as illustrated by the reaction of compds. 5c, 9a and 9b with dimeric (pentamethylcyclopentadienyl) Rh(III) dichloride. The mol. structure of [RhCp*Cl25c] (Cp* = pentamethylcyclopentadienyl) was detd. by x-ray crystallog.
~11 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
193. Preparation of pyrazole compounds as EP1 prostaglandin receptor antagonists
By Conway, Elizabeth Ann; Giblin, Gerard Martin Paul; Gibson, Mairi; Hall, Adrian; Hayhow, Thomas George Christopher; Healy, Mark Patrick; Hurst, David Nigel; Kilford, Ian Reginald; McKeown, Stephen Carl; Naylor, Alan; et al From PCT Int. Appl. (2006), WO 2006114313 A1 20061102, Language: English, Database: CAPLUS

SciFinder® |
Page 139 |
Pyrazoles (shown as I; variables defined below; e.g. 1,1-dimethylethyl [1-[[5-chloro-2-[(phenylmethyl)oxy]phenyl]methyl]- 5-methyl-1H-pyrazol-3-yl]carbamate (1)) or a pharmaceutically acceptable deriv. thereof, a process for the prepn. of such compds., pharmaceutical compns. comprising such compds. and the use of such compds. in medicine are disclosed.
Although the methods of prepn. are not claimed, prepns. and/or characterization data for 500 examples of I are included. For example, 1 was prepd. from 1-[[5-chloro-2-[(phenylmethyl)oxy]phenyl]methyl]-5-methyl-1H-pyrazole-3- carboxylic acid, Et3N, and Ph2P(O)N3 in tBuOH; the starting acid was prepd. by sapon. of the Et ester, which was prepd. by O-benzylation of Et 1-[(5-chloro-2-hydroxyphenyl)methyl]-5-methyl-1H-pyrazole-3-carboxylate (prepn. of similar compd. described). For I: Z is O, S, SO or SO2; Rx is (un)substituted C2-10alkyl, C2-10alkenyl, C2-10alkynyl, CQaQb- heterocyclyl, CQaQb-bicyclic heterocyclyl, or CQaQb-aryl; R1 is CONR3R4, NR3CO2R5, NR3COR6, OCONR3R7, tetrazolyl, oxazolin-2-yl, oxazol-2-yl, benzoxazol-2-yl, pyrrolidinonyl, isoindoledionyl, dihydroisoindolonyl, or (un)substituted SO2NHCOaryl; or R1 is (un)substituted imidazolyl or 1,2,4-triazolyl wherein optionally the imidazole or 1,2,4-triazole ring is fused to give an (un)substituted bicyclic or tricyclic ring system; or R1 = 4-R9-2-oxopiperazin-1-yl. R2a and R2b = H, halo, CN, SO2alkyl, SR3, NO2, or (un)substituted alkyl or alkoxy; R3 is H or C1-4alkyl; R4 is H, OH, (un)substituted alkyl, aryl, heterocyclyl, bicyclic heterocyclyl, CQcQdaryl, CQcQdheterocyclyl, or CQcQdbicyclic heterocyclyl, or SO2R8; R5 is (un)substituted C1-4alkyl, substituted cyclohexyl, Ph, et al.; R6 is alkyl or (un)substituted aryl, heterocyclyl, bicyclic heterocyclyl, CQcQd-Y-aryl, CQcQd-Y-heterocyclyl or CQcQd-Y-bicyclic heterocyclyl; R7 is (un)substituted alkyl, alkenyl, aryl, or CQcQdaryl; R8 is (un)substituted alkyl, aryl or heterocyclyl. R9 is (un)substituted alkyl, alkenyl, (un)substituted CQcQd-Y-aryl, (un)substituted CQcQd-Y-heterocyclyl or (un)substituted CQcQd-Y-bicyclic heterocyclyl; R10 and R11 = H, F and alkyl; or R10 and R11 together with the C to which they are attached form a cycloalkyl ring, optionally contg. up to one heteroatom = O, S, NH or N-alkyl; and Y is CH2 or a bond; Qa and Qb = H, CH3 and F; Qc and Qd = H, CH3 and F; addnl. details including provisos are given in the claims. Results are summarized for a binding assay for the human prostanoid EP1 receptor, a human EP1 Ca mobilization assay and for a human EP3 Ca mobilization assay for many examples of I.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
194. Preparation of iodophenyl azolyl sulfonylcarboxamides as herbicides and plant growth regulators.
By Waldraff, Christian; Dietrich, Hansjoerg; Kehne, Heinz; Hills, Martin; Auler, Thomas; Mueller, Klaus-Helmut; Feucht, Dieter
From PCT Int. Appl. (2006), WO 2006114221 A1 20061102, Language: German, Database: CAPLUS
Title compds. [I; R = OH, cyano, SF5, NO2, NH2, (substituted) hydrocarbyl, hydrocarbyloxy, heterocyclyl, heterocyclyloxy, etc.; m = 0-3; R1 = halo, OH, SH, C-free N residue, C-contg. residue; R2 = (substituted) 5-membered heterocyclyl; W = O, S], were prepd. Thus, 2-iodo-6-methoxybenzenesulfonamide (prepn. given) in MeCN was treated with 4-cyclopropyl- 3-ethoxy-5-oxo-1-phenylcarbonyl-4,5-dihydro-1H-1,2,4-triazole and then with DBU followed by stirring for 60 min. to give 62% 4-cyclopropyl-3-ethoxy-N-[(2-iodo-6-methoxyphenyl)sulfonyl]-5-oxo-4,5-dihydro-1H-1,2,4-triazole-1-carboxamide. The latter and many addnl. I showed very good preemergent herbicidal activity against e.g. Sinapis alba, Avena sativa, Stellaria media, Setaria viridis, etc.
~2 Citings
SciFinder® |
Page 140 |
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
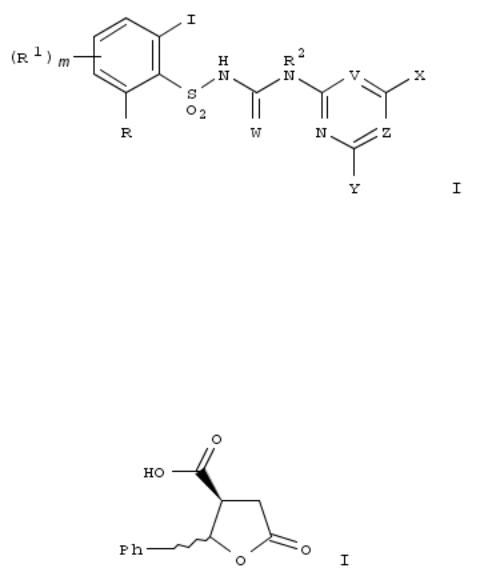
195. Preparation of iodophenyl azinyl sulfonylureas as herbicides and plant growth regulators.
By Waldraff, Christian; Dietrich, Hansjoerg; Ort, Oswald; Kehne, Heinz; Hills, Martin; Auler, Thomas; Mueller, KlausHelmut; Feucht, Dieter
From PCT Int. Appl. (2006), WO 2006114220 A1 20061102, Language: German, Database: CAPLUS
Title compds. [I; R = (substituted) hydrocarbyl, hydrocarbyloxy, O2CR3, SOnR3, OSOnR3, F, Br, iodo, OH, CN, NO2, NH2, SF5, NR4R5, Si(R6)3; n = 1, 2; R1 = halo, OH, SH, C-free N-contg. group, group contg. 1-30 C atoms; R0R10 = atoms to form a N-heterocyclic ring; n = 0-3; R2 = H, (substituted) hydrocarbyl, hydrocarbyloxy; R3 = H, cyano, NR4R5, (substituted) hydrocarbyl, hydrocarbyloxy, heterocyclyl, heterocyclyloxy; R4 = R0Q0; R0 = H, (substituted) acyl, hydrocarbyl, heterocyclyl; Q0 = bond, O, N(R10); R10 = H, (substituted) acyl, hydrocarbyl; R5 = H, (substituted) acyl, hydrocarbyl, heterocyclyl; R4R5 = atoms to form N-heterolcyclyl; R6 = (substituted) hydrocarbyl; W = O, S; X, Y = H, halo, (substituted) alkyl alkoxy, alkylthio; V, Z = CH, N], were prepd. Thus, 2-iodo-6-methoxybenzenesulfonamide (prepn. given) in MeCN was treated with N-(4,6-dimethoxypyrimidin-2-yl)carbamic acid Ph ester and then with DBU followed by stirring for 30 min. to give 77% N-[[(4,6-dimethoxypyrimidin-2-yl)amino]carbonyl]-2-iodo-6-methoxybenzenesulfonamide. The latter and other I showed very good preemergent herbicidal activity against eg. Sinapis alba, Avena sativa, Stellaria media, Abutilon theophrasti, etc.
~16 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
196. Chemoenzymatic synthesis of diastereomeric ethyl γ-benzyl paraconates and determination of the absolute configurations of their acids
By Berti, Federico; Felluga, Fulvia; Forzato, Cristina; Furlan, Giada; Nitti, Patrizia; Pitacco, Giuliana; Valentin, Ennio From Tetrahedron: Asymmetry (2006), 17(16), 2344-2353. Language: English, Database: CAPLUS, DOI:10.1016/j.tetasy.2006.08.013
Enantiopure (99% ee) cisand trans-γ-benzylparaconic acids I and their Et esters were synthesized by a procedure involving kinetic enzymic resoln. of the corresponding lactonic esters with α-chymotrypsin (α-CT) with acetone added as a cosolvent. Their abs. configurations were detd. by 1H NMR anal. of their 1-(9-anthryl)-2,2,2-trifluoroethyl esters.
~14 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
