
Reference_01_08_2014_165529
.pdf
SciFinder® |
Page 21 |
The title spiropiperidine compds. represented by general formula [I; ring A - C6-10 aryl, 5- to 10-membered heterocyclic
ring; Z = O, N R9, S(O)m, CH2 C(O); R9 = H, C1-6 alkyl, C1-7 alkanoyl, C1-6 alkylsulfonyl; bond a or b = a single or double
bond; m = an integer of 0-2; R1-R4 = absent or H, halo, C1-6 alkyl, carboxy-C1-6 alkyl, C1-6 alkoxycarbonyl-C1-6 alkyl, (un)substituted 4- to 10-membered heterocyclyl-C1-6 alkyl, C1-6 alkoxy, C3-18 cycloalkyl-C1-6 alkoxy, C1-6 alkylsulfonyl, C1-6 alkylsulfonyloxy, C1-6 alkylsulfonylamino, HO, cyano, NO2, (CR10R11)lS(O)nR12, Q; R10, R11 = H, halo, C1-6 alkyl; R12 = C1- 20 alkyl, C2-6 alkenyl, halo-C1-6 alkyl, C2-7 alkanoyl, C6-10 aryl, (un)substituted 4- to 10-membered heterocyclyl, C3-10 cycloalkyl-C1-6 alkyl, cyano-C1-6 alkyl, C1-6 alkoxy-C1-6 alkyl, etc.; l = an integer of 1-3; n = an integer of 0-2; R5-R7 = H, halo, cyano, CO2H; R8 = C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, halo- C1-6 alkyl, trialkylsilyl-C1-6 alkyl, cyano-C1-6 alkyl, C1-6 alkoxy- C1-6 alkyl, (un)substituted C3-10 cycloalkyl, etc.; R13, R14 = H, halo; R15 = H or HO or when the bond a is a double bond, R15 is oxo], salts thereof, or solvates of the compds. or the salts. These compds. have an effect of promoting insulin secretion from pancreatic β cells and an effect of lowering blood sugar and are useful as prophylactic and/or therapeutic agents for diseases assocd. with high blood sugar, such as diabetes. Thus, coupling of tert-Bu 6-(4,4,5,5-tetramethyl-1,3,2- dioxaborolan-2-yl)spiro[chromane-2,4'-piperidine]-1'- carboxylate with 5-bromo-2-((methylsulfonyl)methyl)benzonitrile in the presence of tetrakistriphneylphosphine palladium and Na2CO3 in a 1:1 mixt. of 1,4-dioxane and water at 95° for 11 h under argon atm. gave tert-Bu 6-[3-cyano-4- [(methylsulfonyl)methyl]phenyl]spiro[chromane-2,4'-piperidine]- 1'-carboxylate (II). II in vitro promoted the secretion of insulin from hamster pancreatic β cells (HIT-T15 cells) with EC50 of 0.3 nM.
SciFinder® |
Page 22 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
24. CF3SO3H-SiO2 as catalyst for Ferrier rearrangement: an efficient procedure for the synthesis of pseudoglycosides
By Chen, Peiran; Wang, Shaoshan
From Tetrahedron (2013), 69(2), 583-588. Language: English, Database: CAPLUS, DOI:10.1016/j.tet.2012.11.019
An efficient method for the conversion of 2,4,6-tri-O-acetyl-D-glucal to 2,3-unsatd. glycosides by using triflic acid on SiO2 as catalyst has been established. A series of 2,3-unsatd. glucosides were synthesized in good yield and high anomeric selectivity under transition metal-free conditions.
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
25. Preparation of Regioselectively Modified Amylose Derivatives and Their Applications in Chiral HPLC
By Sun, Baishen; Li, Xiaofang; Jin, Zhaolei; Tian, Lili; Wang, Fang; Liu, Guihua; Tang, Shouwan; Pan, Fuyou
From Chromatographia (2012), 75(23-24), 1347-1354. Language: English, Database: CAPLUS, DOI:10.1007/s10337- 012-2338-x
Four regioselectively modified amylose derivs. with three different substituents at the 2-, 3-, and 6-positions were prepd. and their enantiosepns. in HPLC were examd. The nature as well as the arrangement of the substituents significantly influenced their enantiosepns. and each deriv. exhibited characteristic chiral recognition. Amylose 2-benzoyl-3-(3,5- dimethylphenylcarbamate or 3,5-dichlorophenylcarbamate)-6-((S)-1-phenylethylcarbamate) exhibited chiral resolving abilities comparable to the com. available amylose tris(3,5-dimethylphenylcarbamate)-based column, Chiralpak AD and the racemic compds. shown in this study were most effectively resolved on these two derivs. The influence of mobile phase on chiral resoln. was also examd.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
26. Synthesis of γ-fluoroalkylated allylic amine derivatives via palladium-catalyzed Overman rearrangement
By Jiang, Xin-Yi; Chu, Lingling; Wang, Ruo-Wen; Qing, Feng-Ling
From Tetrahedron Letters (2012), 53(50), 6853-6857. Language: English, Database: CAPLUS, DOI:10.1016/j.tetlet.2012.10.045
A Pd-catalyzed Overman rearrangement of α-fluoroalkylated allylic trichloroacetimidates has been developed. This reaction allows for an efficient synthesis of γ-fluoroalkylated allylic amine derivs. with excellent regioand stereoselectivities under mild conditions.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
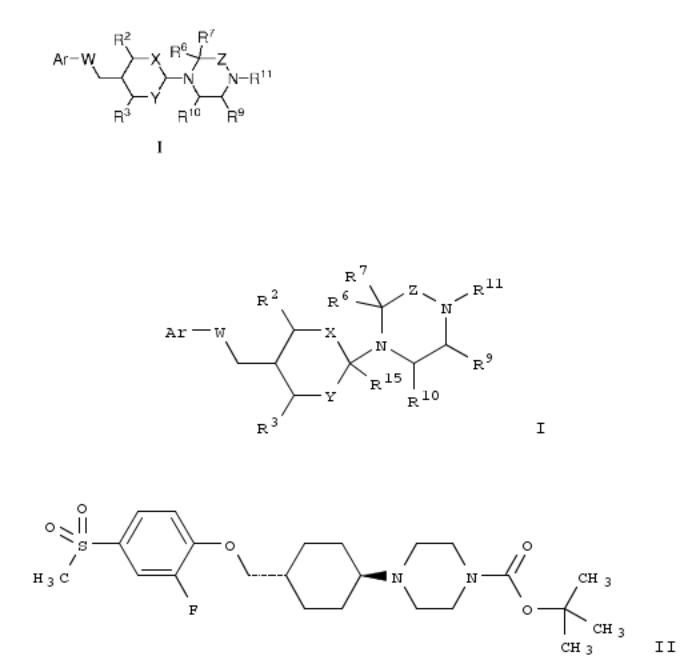
27. Piperazine derivatives as GPR119 receptor modulators and their preparation and use for the treatment of GPR119 receptor-related diseases
By Jones, Robert M.; Buzard, Daniel J.; Han, Sangdon; Kim, Sun Hee; Lehmann, Juerg; Yue, Dawei; Zhu, Xiuwen From PCT Int. Appl. (2012), WO 2012145361 A1 20121026, Language: English, Database: CAPLUS
SciFinder® |
Page 23 |
The invention relates to piperazine derivs. of formula I, which are GPR119 receptor modulators and which are useful in the treatment of GPR119 receptor-related diseases. Compds. of formula I wherein Ar is (un)substituted aryl and (un)substituted heteroaryl; W is absent, O and NH and derivs.; R2 and R3 are H; R2R3 taken together to form CH2CH2; X is absent, CH2CH2, CO, etc.; Y is absent and CH2; Z is CH2CH2, CH2, CH-C1-6 alkyl and CO; R6, R9 and R10 are H; R7 and R8 are independently H and C1-6 alkyl; R6R7, R7R10, R8R9, R8R10 independently taken together to form CH2CH2; R9R10 taken together to form CH2; R11 is (un)substituted C1-6 alkoxycarbonyl, (un)substituted C1-6 alkylsulfonyl, (un)substituted C1-6 alkylthiocarbonyl, etc.; mR15 is H and CN; and pharmaceutically acceptable salts, solvates and hydrates thereof, are claimed. Example compd. II was prepd. by a multistep procedure (procedure given). All the invention compds. were evaluated for their GPR119 receptor modulatory activity (some data given).
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
28. Synthesis of chiral ketones for use in asymmetric epoxidation reactions
By Hayter, Barry R.
From No Corporate Source data available (1998), No pp.. Language: English, Database: CAPLUS
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
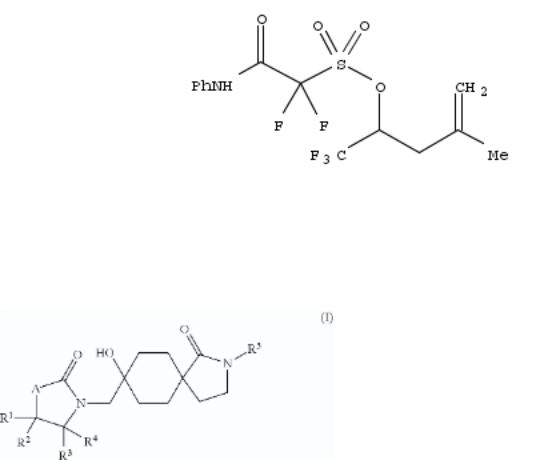
29. Stabilized sulfonic acid amplifiers for photolithography
By Brainard, Robert L.; Cardineau, Brian
From PCT Int. Appl. (2012), WO 2012135286 A2 20121004, Language: English, Database: CAPLUS
SciFinder® |
Page 24 |
Polymeric and nonpolymeric sulfonic acid amplifiers are stabilized by ester or groups, so that in activation with acid the amplifiers decomp. and release the acid. A typical polymer amplifier precursor was manufd. by radical polymn. of 4- hydroxystyrene 0.684, styrene 0.197, 2-methyl-2-adamantyl methacrylate 0.445, and 1,1,1-trifluoro-4-methyl-4-penten-2- yl 1,1-difluoro-2-oxo-2-(4-vinyphenylamino)ethanesulfonate 0.206 g.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
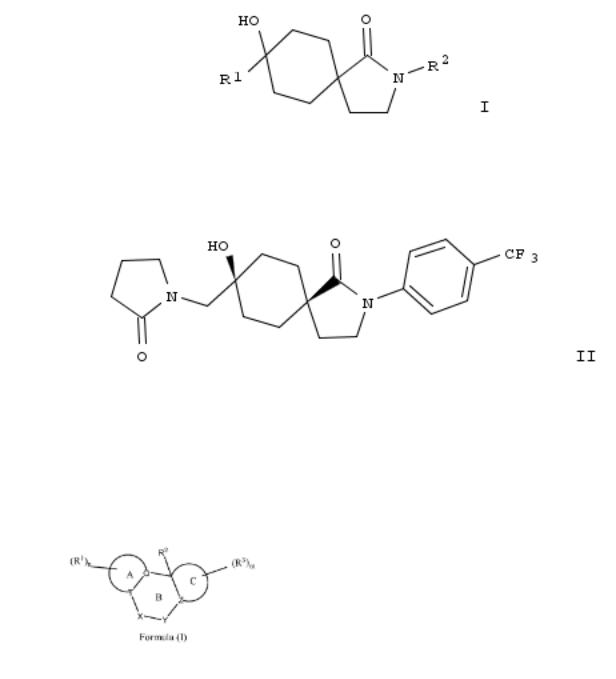
30. Preparation of azaspirodecanone compounds as inhibitors of hormone sensitive lipase (HSL)
By Hunziker, Daniel; Neidhart, Werner
From U.S. Pat. Appl. Publ. (2012), US 20120245191 A1 20120927, Language: English, Database: CAPLUS
Title compds. I [R1 = H, alkyl or cycloalkyl; R2 = H, alkyl or cycloalkyl; R3 = H, alkyl or cycloalkyl; R4 = H, alkyl or cycloalkyl; or R3 and R4 together with the carbon they are attached to form a cycloalkyl; R5 = substituted Ph or pyridinyl; A = C(R6R7), NR8, O or S; R6 = H, alkyl, cycloalkyl, hydroxy, alkoxy or cycloalkoxy; R7 = H, alkyl or cycloalkyl; R8 = H, alkyl or cycloalkyl], and their pharmaceutically acceptable salts, are prepd. and disclosed. Thus, e.g., II was prepd. in 7 steps starting from Et cyclohexanone-4-carboxylate with ethylene glycol. II exhibited IC50 value of 0.0149 μM in human hormonesensitive lipase (HSL) enzyme inhibition assay.

SciFinder® |
Page 25 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
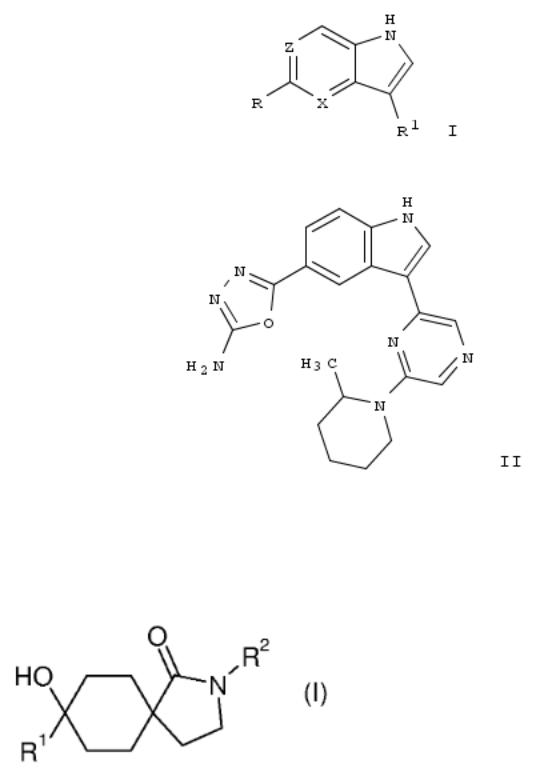
31. Indoles and related compounds as PIM kinase inhibitors and their preparation and use for the treatment of PIM kinase-related diseases
By Wang, Hui-Ling; Cee, Victor C.; Herberich, Bradley J.; Jackson, Claire L. M.; Lanman, Brian Alan; Nixey, Thomas; Pettus, Liping H.; Reed, Anthony B.; Wu, Bin; Wurz, Ryan; et al
From PCT Int. Appl. (2012), WO 2012129338 A1 20120927, Language: English, Database: CAPLUS
The invention relates to indoles and related compds. of formula I, which are PIM kinase inhibitors and which are useful in the treatment of PIM kinase-related diseases. Compds. of formula I wherein X is CH and N; Z is CR2 and N; R is (un)substituted oxazolyl, (un)substituted thiazolyl, (un)substituted thiadiazolyl and (un)substituted oxadiazolyl; R1 is NHCORa, CONHRa, (un)substituted Ph, etc.; R2 is H and halo; Ra is alkyl, (un)substituted cycloalkyl, (un)substituted Ph, etc.; and pharmaceutically acceptable salts thereof, are claimed. Example compd. II•TFA was prepd. by a multistep procedure (procedure given). All the invention compds. were evaluated for their PIM inhibitory activity. From the assay, it was detd. that compd. II•TFA exhibited IC50 values of 0.714 nM, 2.063 nM and 1.061 nM towards PIM-1, PIM-2 and PIM-3, resp.
SciFinder® |
Page 26 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
32. Preparation of azaspirodecanone derivatives as inhibitors of hormone sensitive lipase (HSL)
By Hunziker, Daniel; Neidhart, Werner
From PCT Int. Appl. (2012), WO 2012123468 A1 20120920, Language: English, Database: CAPLUS
Title compds. I [R1 = haloalkoxyalkyl, oxopyrrolidinylalkyl or oxopiperidinylalkyl; R2 = substituted Ph or pyridinyl], and their pharmaceutically acceptable salts or esters, are prepd. as inhibitors of hormone sensitive lipase (HSL). Thus, e.g., compd. II was prepd. by the reaction of Et cyclohexanone-4- carboxylate with ethylene glycol followed by introduction of a cyanomethyl group, subsequent hydrogenation and ring closure to provide 1,4-dioxa-10-aza-dispiro[4.2.4.2]tetradecan- 9-one, which underwent coupling with 1-bromo-4- (trifluoromethyl)benzene, hydrolysis of the cyclic ketal, epoxidn. and coupling with 2-pyrrolidone. Compd. II exhibited inhibitory activity against human HSL with IC50 value of 0.0077 μM. The invention compds. are useful for the treatment of HSL-assocd. diseases such as diabetes, cardiovascular disease, nonalcoholic fatty liver disease, etc.
SciFinder® |
Page 27 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
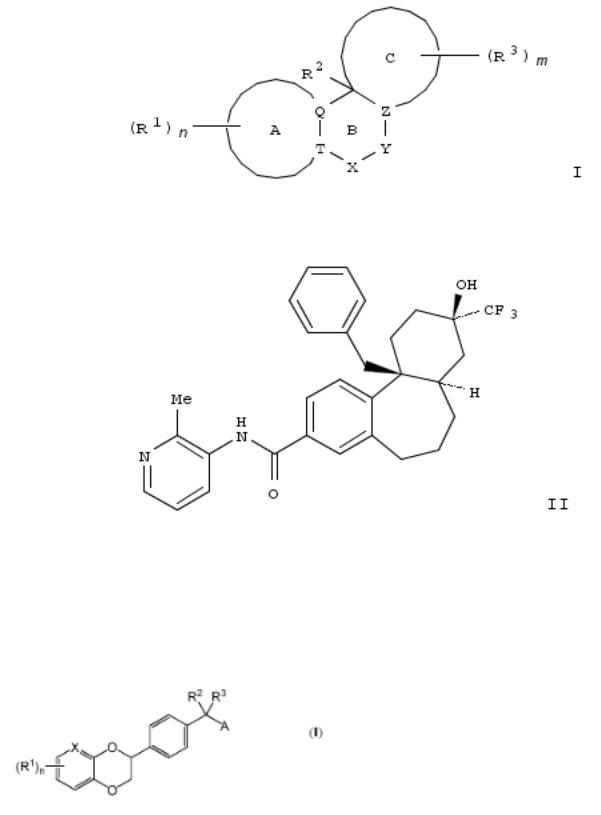
33. Preparation of tricyclic compounds as nuclear hormone receptor modulators
By Cusack, Kevin P.; Gordon, Thomas D.; Ihle, David C.; Hayes, Martin E.; Breinlinger, Eric C.; Ericsson, Anna M.; Li, Bin; Wang, Lei; Martinez, Gloria Y.; Burchat, Andrew; et al
From PCT Int. Appl. (2012), WO 2012125797 A1 20120920, Language: English, Database: CAPLUS
The invention provides tricyclic compds. of formula I and their pharmaceutically acceptable salts, prodrugs, biol. active metabolites, stereoisomers and isomers thereof as nuclear hormone receptor modulators; their prepn. and use in the treatment of immunol. and oncol. conditions. Compds. of formula I wherein ring A is (un)substituted aryl, (un)substituted (un)satd. C5-6 carbocyclyl and (un)substituted heteroaryl; ring C is (un)substituted (un)satd. C5-6 carbocyclyl and (un)substituted heterocyclyl; Q and T are independently C and N provided that both are not N; ring B is 6- to 7-membered ring; X is CO, O, S, etc.; Y is C(R5)2C(R5)2, CR5C(R5)2, COO, etc.; Z is CR4 and N; R1 is H, Br, Cl, F, etc.; R2 is (un)substituted (CH2)yaryl, (un)substituted (CH2)yC1-3 alkyl, (un)substituted heteroaryl, etc.; R3 is H, D, CD3, CF3, etc.; R4 is H, (un)substituted C1-3 alkyl, OH, etc.; R5 is H, F, (un)substituted C3-6 cycloalkyl, etc.; m = 1, 2, 3 and 4; n is 1, 2, 3 and 4; y is 0, 1 and 2; and their pharmaceutically acceptable salts, prodrugs, biol. active metabolites, stereoisomers and isomers, are claimed. Example compd. rac-II was prepd. by a multistep procedure (procedure given). All the invention compds. were evaluated for their nuclear hormone receptor modulatory activity. From the assay, it was detd. that example compd. II exhibited IC50 value of less than 0.1 μM.
SciFinder® |
Page 28 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
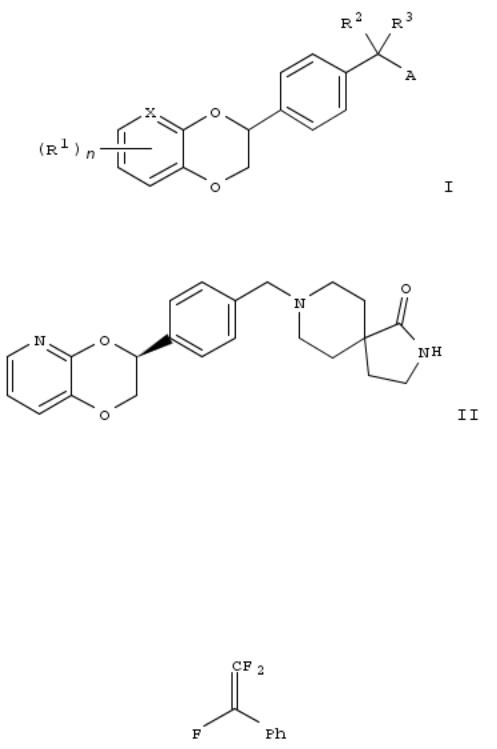
34. Preparation of benzodioxane derivatives as inhibitors of leukotriene A4 hydrolase (LTA4H)
By Abeywardane, Asitha; Burke, Michael J.; Kirrane, Thomas Martin, Jr.; Netherton, Matthew Russell; Padyana, Anil Kumar; Smith Keenan, Lana Louise; Takahashi, Hidenori; Turner, Michael Robert; Zhang, Qiang; Zhang, Qing From PCT Int. Appl. (2012), WO 2012125598 A1 20120920, Language: English, Database: CAPLUS
Title compds. I [X = N or CH; n = 0-3; R1 = halo, OH, CN, alkyl, or cycloalkyl; R2 and R3 = independently H, alkyl or (un)substituted 3- to 6-membered ring when taken together; A = NR4R5; R4 and R5 = independently H, (un)substituted alkyl, cycloalkyl, heterocycloalkyl, aryl or heteroaryl], and their pharmaceutically acceptable salts, are prepd. as inhibitors of leukotriene A4 hydrolase (LTA4H). Thus, e.g., compd. II was prepd. by the reaction of (S)-4-(2,3-dihydro-[1,4]dioxino[2,3- b]pyridin-3-yl)benzaldehyde (prepn. given) with 2,8-diaza- spiro[4.5]decan-1-one hydrochloride in the presence of sodium acetoxyborohydride. Compd. II exhibited inhibitory activity against human LTA4H enzyme with IC50 value of 0.16 nM. The invention compds. are useful for the treatment of leukotrienemediated disorders.
SciFinder® |
Page 29 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
35. Industrial manufacture of high-purity α,β,β-trifluorostyrenes
By Ishii, Akihiro; Ishimaru, Takehisa; Yasumoto, Manabu
From Jpn. Kokai Tokkyo Koho (2012), JP 2012171948 A 20120910, Language: Japanese, Database: CAPLUS
CF2:CFAr (I; Ar = aryl) are manufd. by dehydrofluorination of CF3CFHAr (Ar = same as above) with (Me3Si)2NM (M = alkali metal), and treatment of the reaction mixts. contg. crude I and byproduct (Me3Si)2NH with HF, or HF-org. base salts or complexes. Thus, CF3CFHPh was treated with (Me3Si)2NLi at room temp. for 4 h 30 min, and treated with Bu3N.3HF under ice cooling for 1 h, and at room temp. for 1 h 30 min, filtered, washed with hexane, and distd. to give 86% CF2:CFPh with purity 99.1% measured by GC.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
36. Chiral separation on sulfonated cellulose tris(3,5-dimethylphenylcarbamate)-coated zirconia monolith by capillary electrochromatography
By Lee, Jeong-Mi; Jang, Myung Duk; Park, Jung Hag
From Bulletin of the Korean Chemical Society (2012), 33(8), 2651-2656. Language: English, Database: CAPLUS, DOI:10.5012/bkcs.2012.33.8.2651
SciFinder® |
Page 30 |
Sulfonated cellulose tris(3,5-dimethylphenylcarbamate) (SCDMPC)-coated zirconia monolith (ZM) was used as the chiral stationary phase in capillary electrochromatog. for sepn. of enantiomers of ten chiral compds. in acetonitrile (ACN)- phosphate buffer mixts. as the eluent. Influences of the ACN content, buffer concn. and pH on chiral sepn. were studied. Sepn. data on SCDMPC-ZM were compared with those on CDMPC-ZM. Resoln. factors were better on SCDMPC-ZM than CDMPC-ZM while retention factors were in general shorter on the former than the latter. Best chiral resolns. on SCDMPC-ZM were obtained with the eluent of 50% ACN contg. 50 mM phosphate at pH around 4.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
37. Enantioseparation on helical poly(phenylacetylene)s bearing cinchona alkaloid pendants as chiral stationary phases for HPLC
By Naito, Yuki; Tang, Zhenglin; Iida, Hiroki; Miyabe, Toshitaka; Yashima, Eiji
From Chemistry Letters (2012), 41(8), 809-811. Language: English, Database: CAPLUS, DOI:10.1246/cl.2012.809
Optically active helical poly(phenylacetylene)s bearing cinchona alkaloid pendant groups were coated on macroporous silica gel to obtain novel chiral packing materials for HPLC, which could resolve diverse racemic compds. into enantiomers whose chiral recognition abilities were significantly influenced by the macromol. helicity induced by the alkaloid pendants.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
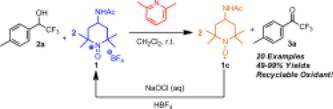
38. Oxidation of α-Trifluoromethyl Alcohols Using a Recyclable Oxoammonium Salt
By Kelly, Christopher B.; Mercadante, Michael A.; Hamlin, Trevor A.; Fletcher, Madison H.; Leadbeater, Nicholas E. From Journal of Organic Chemistry (2012), 77(18), 8131-8141. Language: English, Database: CAPLUS, DOI:10.1021/jo301477s
A simple, mild method for the oxidn. of α-trifluoromethyl alcs. to trifluoromethyl ketones (TFMKs) using the oxoammonium salt 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate is described. Under basic conditions, oxidn. proceeds rapidly and affords good to excellent yields of TFMKs, without concomitant formation of the hydrate. The byproduct of the oxidn., 4-acetylamino-2,2,6,6-tetramethyl-1- piperidinyloxy (1c), is easily recovered and can be conveniently reoxidized to regenerate the oxoammonium salt.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
39. 1,3-Dicarbonyl derivatives of adamantanes and production method thereof
By Akhrem, I. S.; Avetisyan, D. V.; Goryunov, E. I.; Petrovskii, P. V.; Kagramanov, N. D.; Churilova, I. M. From Russ. (2012), RU 2458911 C2 20120820, Language: Russian, Database: CAPLUS
The invention relates to a method of producing 1,3-dicarbonyl derivs. of adamantane I [R = H, X = CF3(CH3)CHO, CHF2CF2CH2O, BrCH2CH2CH2O, C4H3O (furyl); HO, MeO, EtO, iPrO, s-BuO, CF3CH2O, CH≡CCH2O, Et2N, OC4H8N (morpholinyl), PhNH, MeOC6H4; R = Me, X = iPrO, OC4H8N (morpholinyl), C4H3O (furyl), Et2N, PhNH, HO, MeO, MeOC6H4] by carbonylation of an adamantane compd. in the presence of electrophilic catalysts. 1-Bromoadamantane or 1,3-dimethyl-5-bromoadamantane are used as the adamantane compd. and carbonylation is carried out with CO at atm. pressure in a CH2Br2 soln. at temp. 0-25° for 0.5-3 h. A super-electrophilic complex CBr4·2AlBr3 is used as the catalyst with molar ratio [CBr4·2AlBr3]:[adamantane compd.] = (1.2-1.5):1. A nucleophilic substrate is added to the carbonyl deriv. formed in situ without sepn. thereof in a CO atm., said nucleophilic substrate being water or aliph. alc. selected from MeOH, EtOH, i-PrOH, sec-BuOH, fluorineor bromine-contg. alc., or an alc. contg. an acetylene group selected from CF3CH2OH, HOCH(CH3)CF3, HOCH2CF2CF2H, HOCH2CH2CH2Br, HOCH2C=CH; an aliph., cyclic or arom. amine selected from diethylamine, morpholine, aniline; an arom. or heteroarom. hydrocarbon selected from anisole, furan; and the reaction with the nucleophile is carried out at temp. from 0° to 25°.
