
Reference_01_08_2014_165529
.pdf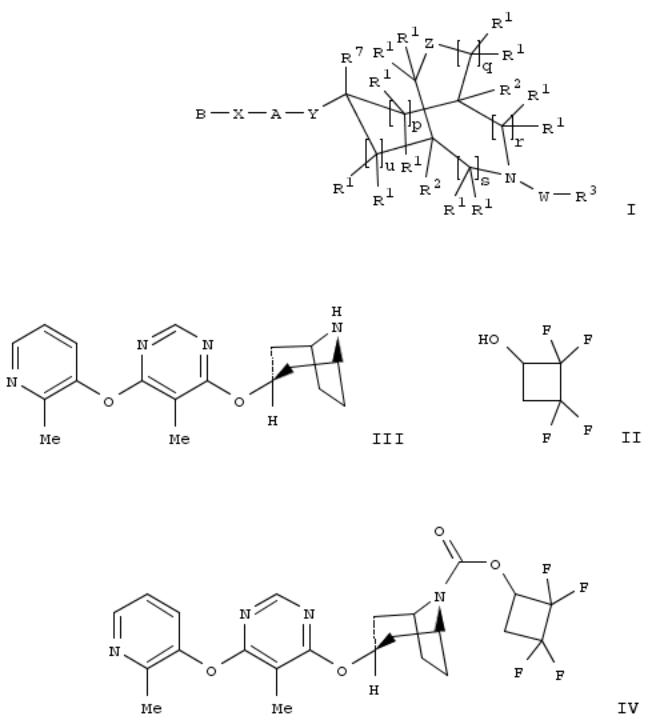
SciFinder® |
Page 111 |
The title compds. I [A = (un)substituted aryl or 5-6 membered heteroaryl; B = (un)substituted (hetero)aryl; W = a bond, alkylene, C(O), C(O)O, etc.; X = C(R1)2, O, NR10, S; Y = O(alkylene), NR10(alkylene), S; Z = a single bond, a double bond, C(O), O, etc.; R1 = H, alkyl, cycloalkyl, etc.; R2 = H or alkyl; R3 = alkyl, alkylene-O-alkyl, alkylene-S-aryl, etc.; R4 = H, alkyl, cycloalkyl, etc.; R7 = H or alkyl; R10 = H, alkyl, hydroxyalkyl, alkoxyalkyl, CO2R4; p, q, r, s, u = 0-2], useful for treating or preventing obesity, diabetes, a metabolic disorder, a cardiovascular disease or a disorder related to the activity of GPR119 in a patient, were prepd. Thus, treating tetrafluorocyclobutyl alc. II with phosgene in the presence of ET3N in CH2Cl2 followed by addn. of compd. III afforded 6% IV. The ability of the exemplified compds. I to activate GPR119 and stimulate increases in cAMP levels was detd. EC50 values were calcd. and ranged from 1 nM to about 20 μM. Pharmaceutical compns. comprising the compd. I, alone or in combination with other therapeutic agent, were disclosed.
~19 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.

SciFinder® |
Page 112 |
151. Asymmetric synthesis of (R)- and (S)-4-methyloctanoic acids. A new route to chiral fatty acids with remote stereocenters
By Munoz, Lourdes; Bosch, M. Pilar; Rosell, Gloria; Guerrero, Angel
From Tetrahedron: Asymmetry (2009), 20(4), 420-424. Language: English, Database: CAPLUS, DOI:10.1016/j.tetasy.2009.02.041
The enantioselective synthesis of both enantiomers of 4-methyloctanoic acid, one major aggregation pheromone component of the rhinoceros beetles of the genus Oryctes and an important aroma compd., is described. The key step of the synthesis is based on a stereospecific alkylation with an alc.-protected alkyl iodide using a pseudoephedrine deriv. as a chiral auxiliary followed by subsequent removal of the auxiliary. Both enantiomers are obtained in excellent yields and enantioselectivities (93-94% ee). The strategy outlined allows prepn. of a wide variety of enantiopure methylbranched satd. and unsatd. fatty acids.
~6 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
152. Synthesis of optically active α-benzyl paraconic acids and their esters and assignment of their absolute configuration
By Berti, Federico; Forzato, Cristina; Furlan, Giada; Nitti, Patrizia; Pitacco, Giuliana; Valentin, Ennio; Zangrando, Ennio From Tetrahedron: Asymmetry (2009), 20(3), 313-321. Language: English, Database: CAPLUS, DOI:10.1016/j.tetasy.2009.01.027
The cisand trans-4-benzylparaconic acids I (R = CH2Ph, R1 = H; R = H, R1 = CH2Ph) and their Et esters were synthesized with high enantiomeric excess by hydrolysis of the corresponding diastereomeric lactonic esters using α- chymotrypsin. Thus, at low conversion values, cisand trans-4-benzyl-5-oxo-3-tetrahydrofurancarboxylic acids were sep. isolated with 99% ee and 92% ee, resp. Both Et ester diastereomers were also obtained in enantiopure form. The abs. configuration of the trans-lactonic acid was assigned by 1H NMR anal. of its ester derivs. with both enantiomers of 1-(9- anthryl)-2,2,2-trifluoroethanol, while that of the cis-lactonic acid was assigned by means of X-ray anal. of a cryst. deriv. The CD curves of the products obtained are also reported.
~7 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
153. Nucleophilic displacements of non-racemic α-trifluoromethyl benzylic triflates
By Hughes, Greg; O'Shea, Paul; Goll, Julie; Gauvreau, Danny; Steele, Jennifer
From Tetrahedron (2009), 65(16), 3189-3196. Language: English, Database: CAPLUS, DOI:10.1016/j.tet.2008.12.064
Effective protocols for the introduction of chiral α-trifluoromethyl benzyl moieties by nucleophilic displacement of enantiomerically enriched α-trifluoromethyl benzylic triflates are presented. The effects of substrate electronics, solvent polarity, temp., and base are studied by measuring the diastereomeric or enantiomeric excesses of the displacement products formed by coupling a variety of α-trifluoromethyl benzylic triflates with a range of nucleophiles including amines, carboxylates, thiols, and malonates. Preliminary investigations to elucidate the mechanism(s) involved in the loss of stereochem. integrity at the benzylic center in the nucleophilic displacement reactions are also reported.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
154. Preparation of oxadiazole compounds as S1P1 agonists
By Harada, Hironori; Hattori, Kazuyuki; Fujita, Kazuya; Morita, Masataka; Imada, Sunao; Abe, Yoshito; Itani, Hiromichi; Morokata, Tatsuaki; Tsutsumi, Hideo
From U.S. Pat. Appl. Publ. (2009), US 20090076070 A1 20090319, Language: English, Database: CAPLUS
SciFinder® |
Page 113 |
Title compds. I [ring A = Q1, etc.; X = single bond, CH2, NR3, etc.; R1 = H, halo, aryl, etc.; R2 = CN, O(alkyl), CHO, etc.; R3 = H; R3 and R1, together with the nitrogen to which they are attached, may form morpholino, pyrrolidino, etc.; when X = single bond, R1 and R2 may combine to form a 5-membered ring (wherein 5-membered ring is optionally substituted with alkyl); R4 = Q2, etc. (one bond from Q2 is linked to oxadiazolyl ring); R5 = H, CN, NHRx, etc.; Rx = H, OH, (un)protected amino, etc.] which have an excellent S1P1 agonist activity, were prepd. For example, reaction of 1,3- difluoropropan-2-ol with NaH followed by in-situ treatment with 2-[4-[5-(3-chloro-4-fluorophenyl)-1,2,4-oxadiazol-3-yl]-1H- indol-1-yl]acetamide afforded compd. II which showed the S1P1 agonistic activity with EC50 = 1.2 nM. Since the compd. I have an S1P1 agonist activity, they are useful as an active ingredients for a treating or preventing a disease caused by unfavorable lymphocytic infiltration, for example, an autoimmune disease such as graft rejection in the transplantation of an organ, bone marrow, or a tissue, a graft-vs.-host disease, rheumatic arthritis, multiple sclerosis, systemic lupus erythematosus, a nephrotic syndrome, encephalomeningitis, myasthenia gravis, pancreatitis, hepatitis, nephritis, diabetes, pulmonary disorder, asthma, atopic dermatitis, inflammatory bowel disease, atherosclerosis, ischemiareperfusion injury, or an inflammatory disease, and further, a disease caused by the abnormal growth or accumulation of cells such as cancer and leukemia. Pharmaceutical compn. comprising the compds. I is disclosed.

SciFinder® |
Page 114 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
155. Nonenzymatic kinetic resolution of racemic 2,2,2-trifluoro-1-aryl ethanol via enantioselective acylation
By Xu, Qing; Zhou, Hui; Geng, Xiaohong; Chen, Peiran
From Tetrahedron (2009), 65(11), 2232-2238. Language: English, Database: CAPLUS, DOI:10.1016/j.tet.2009.01.058

SciFinder® |
Page 115 |
Kinetic resoln. (KR) of a series of 2,2,2-trifluoro-1-aryl ethanols (aryl = Ph, 2-MeC6H4, 4-O2NC6H4, 2-naphthyl, 2-thienyl, etc) with (R)-benzotetramisole I as catalyst was investigated. The results showed that when the aryl group in the substrate was a Ph (or a Ph substituted by an electron-donating group) or a naphthyl (an extended phenyl) group, the system gave an s value higher than 20. Preparative KR examples demonstrated the applicability of this method in the prepn. of some enantiomerically pure 2,2,2-trifluoro-1-aryl ethanols or 2,2,2-trifluoro-1-arylethyl isobutyrates.
~29 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
156. Preparation of multicyclic amino acid derivative tryptophan hydroxylase inhibitors and methods of using them for affecting gastrointestinal transit and gastric emptying
By Liu, Qingyun; Zambrowicz, Brian
From PCT Int. Appl. (2009), WO 2009014972 A1 20090129, Language: English, Database: CAPLUS
The invention is related to the prepn. and use of tryptophan hydroxylase (TPH) inhibitors I [A = (un)substituted cycloalkyl, aryl, heterocyclyl; X = a bond, O, S, CO, SO2, NH and derivs., CH2O and derivs., etc.; D = (un)substituted aryl, heterocyclyl; R1, R2 = independently H, (un)substituted alkyl, alkyl/aryl, alkyl/heterocyclyl; Y = (CH2)n; n = 0-3], their pharmaceutically acceptable salts and solvates, in the manuf. of a medicament for slowing gastrointestinal (GI) motility in a patient. Thus, monoamination of 2-amino-4,6-dichloro-1,3,5-triazine with (R)-(+)-1-(2-naphthyl)ethylamine and Pdcoupling of chloride intermediate (no data) with L-p-boronophenylalanine gave II. The potent TPH1 inhibitor slowed GI motility and gastric emptying in a dose-dependent manner when administered to Sprague-Dawley rats. The effects of the compd. on GI transit and gastric emptying correlated with changes in 5-HT levels in the blood and proximal colon.
~2 Citings

SciFinder® |
Page 116 |
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
157. Synthesis and structural characterisation of gallium and indium fluoroalkoxide ate complexes
By Andrews, Philip C.; Forsyth, Craig M.; Junk, Peter C.; Nuzhnaya, Iryna; Spiccia, Leone
From Journal of Organometallic Chemistry (2009), 694(3), 373-381. Language: English, Database: CAPLUS, DOI:10.1016/j.jorganchem.2008.11.002
The treatment of InCl3 with MOCH(CF3)2 (M = Li, Na, K) in a 1:6 stoichiometry, followed by recrystn. gave the bimetallic ate complexes [Na3In(OCH(CF3)2)6(THF)3] (2) and [Li3In(OCH(CF3)2)6(THF)3] (5) from hexane, and [K3In(OCH(CF3)2)6]n
(4) from a THF and toluene mixt. If a 1:3 stoichiometry was used chloride contg. compds. [Na2InCl(OCH(CF3)2)4(THF)4]
(1) and [KInCl2(OCH(CF3)2)2(THF)3]n·THF (3) were obtained on recrystn. from hexane. Treatment of GaCl3 with 6 equiv of LiOCMe2CF3 gives [LiGa(OCMe2CF3)4(THF)2] (6) on recrystn. from hexane. The protolysis reaction between In(N(SiMe3)2)3, formed in situ from (Me3Si)2NH, BuLi and InCl3, and HOCHMeCF3 gave [LiIn(OCHMeCF3)3Bu]2 (7) from hexane. The structures of 2, 4, and 5 all contain the tetranuclear core InO6M3. Compds. 1 and 3 have residual chloride; 1 is a trinuclear species with two THF ligands per Na, while 3 is a linear polymer. Compd. 6 has a GaO2Li fourmembered parallelogram at its core. Complex 7 has a tetranuclear In2O6Li2 core and an unexpected Bu group on the In atoms. The coordination spheres of the alkali metals in 1-6 include solvated THF while 1-5 display addnl. close M···F interactions.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
158. Preparation of pyridones as GPR119 G protein-coupled receptor agonists
By Wacker, Dean A.; Rossi, Karen A.; Wang, Ying
From PCT Int. Appl. (2009), WO 2009012275 A1 20090122, Language: English, Database: CAPLUS
The invention is related to pyridones I and II [G = CH, N; Q = C, N; X = CH, N, provided that Q and X are not both N; Y = CH2, NH and derivs., CO, O, OCH2 and derivs., S(O)0-2; U = (CH2)n; V = (CH2)m; n, m = independently 0-2; Z = (CH2)q; q = 1-2; R1 = (un)substituted 6-membered monocyclic (hetero)/aryl, 5-membered monocyclic heteroaryl; R2 = (un)substituted cycloalkyl, (hetero)/aryl, heterocyclyl, etc.; R20, R21 = independently H, halo, CN, CO2H, OCF3, haloalkyl, etc.] which are GPR119 G protein-coupled receptor modulators, esp. GPR119 G agonists, and are useful in treating, preventing, or slowing the progression of diseases requiring GPR119 G protein-coupled receptor modulator therapy. Thus, arylation of 4-benzyloxy-2(1H)-pyridone with 4-bromophenyl Me sulfone, debenzylation, alkylation of the hydroxypyridinone with tert-Bu 4-[(methylsulfonyl)oxy]piperidine-1-carboxylate (prepn. given) gave III. The in vivo modulation of recombinant human GPR119 was detd. in a HIT-T15 cAMP assay, human Tet-inducible CAMP assay and luciferase assay (some data given). I, alone, or in combination with another therapeutic agent, are useful for treating diabetes, hyperglycemia, impaired glucose tolerance, obesity, metabolic syndrome, etc.

SciFinder® |
Page 117 |
~7 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
159. Development of a Practical Synthesis of a p38 MAP Kinase Inhibitor
By Thiel, Oliver R.; Achmatowicz, Michal; Bernard, Charles; Wheeler, Philip; Savarin, Cecile; Correll, Tiffany L.; Kasparian, Annie; Allgeier, Alan; Bartberger, Michael D.; Tan, Helming; et al
From Organic Process Research & Development (2009), 13(2), 230-241. Language: English, Database: CAPLUS, DOI:10.1021/op800250v
A practical synthesis of the phthalazine-based p38 MAP kinase inhibitor I was needed for an ongoing program. Vibrational CD provided the assignment of the abs. stereochem. of the target compd. The selected synthetic route for I required identification of efficient reaction conditions for the construction of carbonoxygen, carbon-carbon, and carbon-nitrogen bonds to connect the key building blocks. An efficient two-step method (chlorodehydroxylation, arom. nucleophilic substitution) for the synthesis of aryl ether II[(S)-10] was developed. PAT (in situ Raman spectroscopy) was utilized to monitor and control the formation of a lithium alkoxide in this reaction. The synthesis of I was completed using high-yielding Suzukiand amidecoupling reactions. The isolation conditions for these steps were optimized to obtain material of very high purity without the need for any complicated workup procedures.
~9 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 118 |
160. Remarkable substituent effects on the oxidizing ability of tetraarylbismuthonium tetrafluoroborates in alcohol oxidation
By Matano, Yoshihiro; Suzuki, Takeshi; Iwata, Takaharu; Shinokura, Tomonori; Imahori, Hiroshi
From Bulletin of the Chemical Society of Japan (2008), 81(12), 1621-1628. Language: English, Database: CAPLUS, DOI:10.1246/bcsj.81.1621
Substituent effects on the oxidizing ability of tetraarylbismuthonium tetrafluoroborates in alc. oxidn. are reported. Intermol. and intramol. competition expts. on geraniol oxidn. by the combined use of tetraarylbismuthonium
tetrafluoroborates and N,N,N',N'-tetramethylguanidine (TMG) have revealed that the oxidizing ability of the bismuthonium salt increases by the introduction of Me groups at the ortho position and an electron-withdrawing group at the para
position of the aryl ligands. The intermol. and intramol. H/D kinetic isotope effects obsd. for the competitive oxidn. of p- bromobenzyl alcs. have shown that the present oxidn. reaction consists of fast pre-equil. leading to alkoxytetraarylbismuth(V) intermediates (first step) and α-hydrogen abstraction by the aryl ligand attached to the bismuth (second step). The exptl. results demonstrate that the electron-deficient aryl ligands enhance the electrophilicity at the bismuth center to put forward the first step and that the bulky ligands destabilize the alkoxybismuth(V) intermediates to accelerate the second step. The newly explored mesityland 2,6-xylyltriarylbismuthonium salts have proven to convert primary and secondary alcs. to the corresponding carbonyl compds. with high efficiency under mild conditions. A remarkable steric effect of these oxidants has also been exhibited in the chemoselective oxidn. between primary and secondary benzylic alcs.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
161. Pharmaceutical compositions containing multicyclic amino acid derivative tryptophan hydroxylase inhibitors and methods of using them in treatment, prevention and combination therapy of pulmonary hypertension and related diseases
By Sands, Arthur T.
From PCT Int. Appl. (2009), WO 2009009561 A1 20090115, Language: English, Database: CAPLUS
The invention is related to combinations contg. a tryptophan hydroxylase (THP) inhibitor, esp. TPH1 inhibitor I [A = (un)substituted cycloalkyl, aryl, heterocyclyl; X = a bond, O, S, CO, SO2, NH and derivs., CH2O and derivs., etc.; D = (un)substituted aryl, heterocyclyl; R1, R2 = independently H, (un)substituted alkyl, alkyl/aryl, alkyl/heterocyclyl; Y = (CH2)n; n = 0-3] and another active pharmaceutical for treating pulmonary hypertension and related diseases. Thus, monoamination of 2-amino-4,6-dichloro-1,3,5-triazine with (R)-(+)-1-(2-naphthyl)ethylamine and Pd-coupling of chloride intermediate (no data) with L-p-boronophenylalanine gave II. The potent TPH1 inhibitor was found to reduce 5- hydroxytryptamine levels in both the small and large intestine, but not in brain.

SciFinder® |
Page 119 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
162. Preparation of multicyclic amino acid derivations as TPH1 inhibitors for treating serotonin-mediated diseases
By Brown, Philip Manton; Liu, Qingyun; Zambrowicz, Brian
From PCT Int. Appl. (2008), WO 2009002964 A1 20081231, Language: English, Database: CAPLUS
Compds. inhibiting tryptophanhydroxylase (TPH) useful for treating serotonin-mediated diseases and disorders such as e.g. a cardiovascular or pulmonary disease or disorder are disclosed. The invention is related to the use of a compd. inhibiting tryptophan hydroxylase (TPH), esp. TPH1 of formula I [A = (un)substituted cycloalkyl, aryl, heterocyclyl; X = a bond, O, S, CO, SO2, NH and derivs., CH2O and derivs., etc.; D = (un)substituted aryl, heterocyclyl; R1, R2 = independently H, (un)substituted alkyl, alkyl/aryl, alkyl/heterocyclyl; Y = (CH2)n; n = 0-3 ] for treating serotonin-mediated diseases and disorders such as a cardiovascular or pulmonary disease or disorder (no data). Thus, monoamination of 2- amino-4,6-dichloro-1,3,5-triazine with (R)-(+)-1-(2-naphthyl)ethylamine and Pd-coupling of chloride intermediate (no data) with L-p-boronophenylalanine gave II. Potent TPH1 inhibitors I reduced 5-hydroxytryptamine levels in both the small and large intestine, but not in the brain of mice following their oral administration.

SciFinder® |
Page 120 |
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
163. Pharmaceutical compositions containing multicyclic amino acid derivative tryptophan hydroxylase inhibitors and methods of using them for reducing or avoiding serotonin-mediated adverse effects associated with the administration of one or more drugs
By Brown, Philip Manton; Liu, Qingyun
From PCT Int. Appl. (2008), WO 2009002970 A1 20081231, Language: English, Database: CAPLUS
The invention is related to a pharmaceutical compn. contg. a first active pharmaceutical ingredient that has assocd. with it a serotonin-mediated adverse effect and a second active pharmaceutical ingredient that is a tryptophan hydroxylase (TPH) inhibitor, esp. TPH1 inhibitor I [A = (un)substituted cycloalkyl, aryl, heterocyclyl; X = a bond, O, S, CO, SO2, NH and derivs., CH2O and derivs., etc.; D = (un)substituted aryl, heterocyclyl; R1, R2 = independently H, (un)substituted alkyl, alkyl/aryl, alkyl/heterocyclyl; Y = (CH2)n; n = 0-3] and its use for reducing or avoiding serotonin-mediated adverse effects assocd. with the administration of one or more drugs. Thus, monoamination of 2-amino-4,6-dichloro-1,3,5-triazine with (R)-(+)-1-(2-naphthyl)ethylamine and Pd-coupling of chloride intermediate (no data) with L-p-boronophenylalanine gave II. The potent TPH1 inhibitor was found to reduce 5-hydroxytryptamine levels in both the small and large intestine, but not in brain.
