
Reference_01_08_2014_165529
.pdf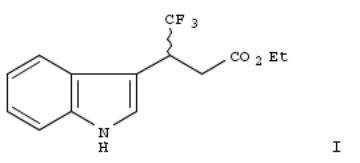
SciFinder® |
Page 181 |
~15 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
278. A facile preparation of enantiomers of ethyl 4,4,4-trifluoro-3-(indole-3-)butyrate, a novel plant growth regulator
By Kato, Katsuya; Katayama, Masato; Gautam, Rakesh K.; Fujii, Shozo; Fukaya, Haruhiko; Kimoto, Hiroshi From Journal of Fermentation and Bioengineering (1995), 79(2), 171-3. Language: English, Database: CAPLUS, DOI:10.1016/0922-338X(95)94087-8
Malonic ester synthesis combined with lipase-catalyzed enantioselective partial hydrolysis was used to prep. enantiomers of Et 4,4,4-trifluoro-3-(indole-3-)butyrate (I), a novel plant growth regulator. Among six com. available enzymes, lipase AK (a lipase from Pseudomonas sp.) showed high enantioselectivity and moderate reactivity. The hydrolysis of racemic di-Et 2,2,2-trifluoro-1-(indole-3-)ethylmalonate with lipase AK gave, following usual treatment of the products, S-(+)-4,4,4-trifluoro-3-(indole-3-)butyric acid (TFIBA) Et ester having 95% e.e. and R-(-)-TFIBA Et ester having >99% e.e. The optimized conditions for lipase AK hydrolysis were 0.1 M phosphate buffer (pH 6.0) contg. 10% hexane at 45°C.
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
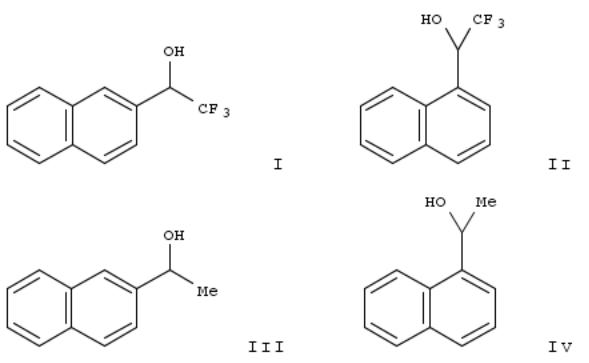
279. Lipase-catalyzed enantioselective synthesis of naphthyl trifluoromethyl carbinols and their corresponding nonfluorinated counterparts
By Gaspar, Jordi; Guerrero, Angel
From Tetrahedron: Asymmetry (1995), 6(1), 231-8. Language: English, Database: CAPLUS, DOI:10.1016/0957- 4166(94)00379-P
Enantioselective synthesis of both enantiomers of β- and α-naphthyl trifluoromethyl carbinols (R)-I1a, (S)-I1a, (R)-II2a,
(S)-II2a has been achieved through acylation of the corresponding racemic alcs. (RS)-I1a and (RS)-II2a with vinyl acetate in the presence of lipase PS. The effect of fluorine atoms on the extent and enantioselectivity of the process has been tested by carrying out the same biocatalytic transformation on their non-fluorinated counterparts (RS)-III1b and (RS)-IV2b. The order of reactivity follows the trend (RS)-III1b > (RS)-IV2b ≈ (RS)-I1a > (RS)-II2a. Effect of the hydrophobicity of the solvent in the resoln. of (RS)-I1a is also presented.
SciFinder® |
Page 182 |
~15 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
280. Imidazole-assisted intramolecular phenoxythiocarbonylation of tertiary alcohols. A key reaction for the deoxygenation of α-trifluoromethylarylmethyl alcohols
By Hsu, Fu-Lian; Zhang, Xiaoyan; Hong, Seoung-Soo; Berg, Frederic J.; Miller, Duane D.
From Heterocycles (1994), 39(2), 801-9. Language: English, Database: CAPLUS, DOI:10.3987/COM-94-S(B)77
The deoxygenation of α-trifluoromethylarylmethyl alcs. failed under catalytic hydrogenation conditions. However, these alcs. can be deoxygenated via their thionocarbonate intermediates followed by homolytic reductive cleavage of the C-O bond. The formation of the Ph thionocarbonate esters is sterically dependent. Consequently, secondary α- trifluoromethyl arylmethyl alcs. can be smoothly converted to thionocarbonates, but tertiary alcs. cannot. Exceptions to this lack of reactivity are the aryl 4-substituted imidazolyl trifluoromethyl carbinols, which do form the thionocarbonates under these conditions.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
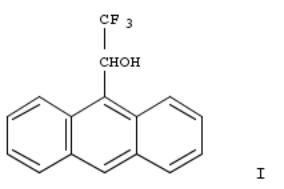
281. Resolution of racemic sterically hindered secondary alcohols via enzymic alcoholysis of their esters. The first enzymic preparation of optically pure 2,2,2-trifluoro-1-(9-anthryl)ethanols
By Shkolnik, Eleonora; Gutman, Arie L.
From Bioorganic & Medicinal Chemistry (1994), 2(7), 567-72. Language: English, Database: CAPLUS, DOI:10.1016/0968-0896(94)85003-8
An approach was developed which exploits the non aq. enzymic alcoholysis reaction for resoln. of racemic sterically hindered secondary alcs. The method was used effectively in the first enzymic prepn. of both enantiomers of the title compd., i.e., (±)-α-(trifluoromethyl)-9-anthracenemethanol (I) via porcine pancreatic lipase-catalyzed alcoholysis of its butyrate ester. A considerable enhancement of the reaction rate was achieved by dispersion of the powd. enzyme prepn. on aluminum oxide. A facile procedure was developed for sepg. (R)-I from the unreactive (S)-butyrate ester and for the hydrolysis of the latter into the (S)-I. The preparative usefulness of the resoln. procedure is demonstrated by the convenience of the scaled-up enzymic expt. carried out on 370 g of substrate in an ordinary flat bottom flask.
SciFinder® |
Page 183 |
~6 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
282. Lipase-catalyzed optical resolution of 2,2,2-trifluoro-1-(indole-3-)ethanols in organic solvents
By Kato, Katsuya; Katayama, Masato; Gautam, Rakesh K.; Fujii, Shozo; Kimoto, Hiroshi
From Journal of Fermentation and Bioengineering (1994), 78(1), 64-9. Language: English, Database: CAPLUS, DOI:10.1016/0922-338X(94)90180-5
As a result of screening 19 enzymes, lipase AK (a lipase from Pseudomonas sp.) was suitable for the optical resoln. of 2,2,2-trifluoro-1-(indole-3-)ethanol (TFIE). The combination of two lipase AK-catalyzed reactions gave S-(+)-TFIE and R- (-)-TFIE with 97% e.e. and 95% e.e., resp. The conditions detd. as optimum for the reactions were in octane at 45° for 8 h, and using vinyl acetate for acetylation and using propanol for alcoholysis. In cases of TFIEs with a Me group on the indole nucleus, the enantioselectivity of lipase AK increases when the Me group is far from the chiral center.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
283. Lipase-catalyzed optical resolution of 2,2,2-trifluoro-1-(1-naphthyl)ethanol
By Kato, Katsuya; Katayama, Masato; Gautam, Rakesh K.; Fujii, Shozo; Kimoto, Hiroshi
From Bioscience, Biotechnology, and Biochemistry (1994), 58(7), 1353-4. Language: English, Database: CAPLUS, DOI:10.1271/bbb.58.1353
The optical resoln. of the title compd. (TFNE) was achieved by lipase LIP-catalyzed enantioselective acetylation (E value >100) with vinyl acetate in octane. S-TFNE acetate and R-TFNE were obtained with high optical purity.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
284. Synthesis of and Analysis of Thiol Additions to β-Alkyl-α,β-unsaturated Trifluoromethyl Ketones
By Linderman, Russell J.; Jamois, Eric A.; Tennyson, Scott D.
From Journal of Organic Chemistry (1994), 59(5), 957-62. Language: English, Database: CAPLUS, DOI:10.1021/jo00084a009
A series of β-alkyl-α,β-unsatd. trifluoromethyl ketones have been prepd. from the corresponding α-acetylenic trifluoromethyl ketones. The addn. of sulfur nucleophiles was shown by chem. and 19F NMR studies to proceed exclusively by 1,4-addn. with no indication of any 1,2-addn. products. The unsatd. trifluoromethyl ketones have been shown to be effective inhibitors of rat cytosolic glutathione-S-transferase.
~22 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
285. Synthetic route to meso-tetrahydrocarbyl or substituted hydrocarbyl porphyrins and derivatives
By Wijesekera, Tilak P.; Wagner, Richard W.
From U.S. (1993), US 5241062 A 19930831, Language: English, Database: CAPLUS

SciFinder® |
Page 184 |
Porphyrins are prepd. by converting the OH group of (hydroxymethyl)pyrroles I [R1 = (un)substituted hydrocarbyl; R2, R3 = H, electron-deficient group] to a better leaving group, then removing the group and cyclizing the resultant species. For example, pyrrole was trifluoroacetylated with (CF3CO)2O in benzene at 2-3° (75%) and the resultant 2-trifluoroacetyl deriv. was reduced by NaBH4 in MeOH at 0-5° (>90%) to give I (R1 = CF3, R2 = R3 = H). This was treated with p- O2NC6H4COCl in Et2O contg. Et3N at <10° to give a p-nitrobenzoate ester, which was too reactive to isolate, but which could be cyclized in situ. Diln. with EtCO2H and treatment with Zn(OAc)2 at reflux gave a mixt. of [mesotetra(trifluoromethyl)porphinato]zinc II (M = Zn) and nonmetallated porphyrin I (M = 2H) in up to 2% overall yield; use of BF3.Et2O in CHCl3 as the dilg. medium gave only the latter product in similar yield.
~12 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
286. Enzymic preparation of both enantiomers of 4,4,4-trifluoro-3-(indole-3-)butyric acid, a novel plant growth regulator
By Kato, Katsuya; Katayama, Masato; Gautam, Rakesh K.; Fujii, Shozo; Kimoto, Hiroshi
From Journal of Fermentation and Bioengineering (1993), 76(3), 178-83. Language: English, Database: CAPLUS, DOI:10.1016/0922-338X(93)90004-R
Efficient optical resoln. of both enantiomers of 4,4,4-trifluoro-3-(indole-3-)butyric acid (TFIBA), a novel plant growth regulator, was achieved by two enzyme-catalyzed steps using lipase AK (a lipase from Pseudomonas sp.). S-(+)-TFIBA and R-(-)-TFIBA Et ester were prepd. with 98% e.e. by the first step of lipase-catalyzed enantioselective hydrolysis in a 0.1 M acetate buffer (pH 5.0) contg. 10% t-BuOH at 55°C for 45 h. The % e.e. in S-(+)-TFIBA was further improved to >99% e.e. by lipase-catalyzed enantioselective esterification with ethanol in heptane and subsequent hydrolysis using PLE-A (an esterase from pig liver). R-(-)-TFIBA was prepd. with >99% e.e. by the second step of lipase-catalyzed enantioselective hydrolysis under the same conditions as described above and subsequent hydrolysis using PLE-A. The root growth-promoting activity of S-(+)-TFIBA was about 10-fold higher than that of R-(-)-TFIBA for black matpe, Chinese cabbage, lettuce, and rice plants (Ohgonbare and Tan-ginbozu).
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
287. Preparation of trifluoromethyl ketones
No Inventor data available
From Ger. Offen. (1993), DE 4201435 A1 19930722, Language: German, Database: CAPLUS
RCOCF3 [R = (substituted) aliph. hydrocarbyl, arom. hydrocrabyl] were prepd. by oxidn. of RCH(OH)CF3 by YOnH (Y = Cl, Br, iodo; n = 1-4) or salts thereof. Thus, PhCH(OH)CF3 were stirred 6 h at room temp. with 12% aq. NaOCl in CH2Cl2 contg. Bu4NHSO4 to give 75% PhCOCF3.

SciFinder® |
Page 185 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
288. Specificity of antibody-catalyzed transesterifications using enol esters: a comparison with lipase reactions
By Fernholz, Erhard; Schloeder, Diane; Liu, Kevin K. C.; Bradshaw, Curt W.; Huang, Hongmei; Janda, Kim; Lerner, Richard A.; Wong, Chi Huey
From Journal of Organic Chemistry (1992), 57(17), 4756-61. Language: English, Database: CAPLUS, DOI:10.1021/jo00043a042
A catalytic antibody elicited against phosphonate PhCHMeOP(O)(O-)CH2C6H4NHCO(CH2)3CO2H-p is studied. The antibody catalyzes not only the hydrolysis of ester (S)-PhCHMeO2CCH2C6H4NHCO(CH2)3CO2H-p, but also the transfer of acyl group from enol ester p-AcNHC6H4CH2CO2CH:CH2 in aq. soln. to a no. of alcs. structurally and sterically related to (S)-1-phenylethanol. Comparison of the antibody reactions to lipase-catalyzed transesterifications indicates that the lipase reactions can only be carried out in org. solvents, and in a representative case the antibody-catalyzed reaction is as effective as the enzymic reaction, both with a kcat 6 min-1. Some esters with high enantiomeric purity were prepd. on mg scales based on the antibody-catalyzed transesterifications in aq. soln. Although a no. of alcs. and esters can be used as substrates for the lipase and antibody reactions, the antibody catalysis is more predictable with regard to the substrate specificity and stereoselectivity.
~7 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
289. Trifluoromethylation of chiral aldehyde and synthesis of 6-deoxy-6,6,6-trifluorohexoses
By Hanzawa, Yuji; Uda, Junichiro; Kobayashi, Yoshiro; Ishido, Yoshiharu; Taguchi, Takeo; Shiro, Motoo From Chemical & Pharmaceutical Bulletin (1991), 39(9), 2459-61. Language: English, Database: CAPLUS, DOI:10.1248/cpb.39.2459
Trifluoromethylation of chiral aldehydes derived from sugars was efficiently carried out by treatment with CF3I-Zn in DMF under ultrasonic irradn. Thus, 6,6,6-trifluoro-L-daunosamine deriv. I was prepd. in 5 steps via (2S,3R)-4,4,4- trifluorobutane-1,2,3-triol deriv. II, which was obtained by trifluoromethylation of 2,3-O-cyclohexylidene-L-glyceraldehyde by this procedure.
~18 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 186 |
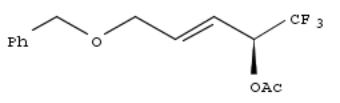
290. Optically active 3-(2-trifluoro-1-hydroxyethyl)propenyl benzyl ether, derivatives thereof, method for preparing the same and use thereof for liquid crystal compound
By Kitazume, Tomoya; Yamazaki, Takashi; Iwatsubo, Hitoshi
From U.S. (1991), US 5047346 A 19910910, Language: English, Database: CAPLUS
A process for the prepn. of benzyl (R)-5,5,5-trifluoro-4-hydroxy-2-pentenyl ether (I) comprises the lipase or esterasecatalyzed esterification of benzyl 5,5,5-trifluoro-4-hydroxy-2-pentenyl ether; the corresponding ester of benzyl (S)-5,5,5- trifluoro-4-hydroxy-2-pentenyl ether (II) is obtained in this process together with I. Hydrolysis of benzyl 5,5,5-trifluoro-4- (acetoxy)-2-pentenyl ether with lipase MY gave 88% optically pure I and remaining II. Compds. like I may be acylated with a BzOH deriv. to give liq.-crystal compds. having 1-4 phenylene groups and a trifluoromethyl group. Acylation of 1,1,1-trifluoro-2-octanol with 4-PhCH2C6H4COCl gave 1,1,1-trifluoro-2-octyl 4-(benzyloxy)benzoate; deprotection and subsequent acylation with 4'-octylbiphenylyl-4-carboxylate gave 4-[[(1,1,1-trifluoro-2-octyl)oxy]carbonyl]phenyl] 4'- (octyloxy)biphenylyl-4-carboxylate.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
291. Studies on trifluoromethyl ketones. VII. Ene reaction of trifluoroacetaldehyde and its application for synthesis of trifluoromethyl compounds
By Ogawa, Keizo; Nagai, Takabumi; Nonomura, Masahiko; Takagi, Toshiyuki; Koyama, Mayumi; Ando, Akira; Miki, Takuichi; Kumadaki, Itsumaro
From Chemical & Pharmaceutical Bulletin (1991), 39(7), 1707-12. Language: English, Database: CAPLUS, DOI:10.1248/cpb.39.1707
The ene reaction of trifluoroacetaldehyde was examd. The aldehyde reacted with various ene compds. as a good enophile in the presence of Lewis acids, among which methylaluminum dichloride worked best, though polymn. of the aldehyde caused by the Lewis acid often lowered the isolated yields of the ene products. The ene reaction products were successfully oxidized to trifluoromethyl β,γ-unsatd. ketones with Dess-Martin reagent. Redn. of the ene reaction products followed by oxidn. with Jones reagent gave satd. trifluoromethyl ketones. The β,γ-unsatd. ketone rearranged on thermolysis to an α,β-unsatd. ketone. These ketones obtained were converted to other types of trifluoromethyl compds. Thus, the ene reaction of trifluoroacetaldehyde provides a versatile method for synthesis of many types of trifluoromethyl compds. During this derivatization, a trifluoromethyl group was found to behave as a much larger substituent than a decyl group.
~10 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
292. Electron-donating ability of the cyclopropyl substituent in the solvolyses of an α-CF3-substituted secondary-alkyl tosylate
By Roberts, Donald D.
From Journal of Organic Chemistry (1991), 56(19), 5661-5. Language: English, Database: CAPLUS,
DOI:10.1021/jo00019a037
The solvolysis of cyclopropyl(trifluoromethyl)carbinyl tosylate (I) have been detd. in a series of aq. alc., aq. trifluoroethanol, and carboxylic acid solvents. Anal. of the rate data for % internal-return isomerization and salt effects and correlation with YOTs values indicate that I underwent solvolysis by the k pathway. Comparison of the relative ability of cyclopropyl and Ph groups to stabilized carbocation-like transition states in solvolysis reactions of the secondary systems RCH(Y)CH3 and RCH(Y)CF3 reveals that replacement of Ph with cyclopropyl increases the rate in both systems by a factor of 102.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
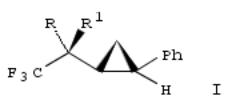
293. A highly stereocontrolled synthesis of α-hydroxycyclopropanes possessing a trifluoromethyl group
SciFinder® |
Page 187 |
By Yamazaki, Takashi; Lin, Jenq Tain; Takeda, Mitsunori; Kitazume, Tomoya
From Tetrahedron: Asymmetry (1990), 1(6), 351-4. Language: English, Database: CAPLUS, DOI:10.1016/0957- 4166(90)90031-5
The stereoselective Sm-promoted cyclopropanation of (R)- or (S)-F3CCH(OH)CH:CRR1 (R, R1 = H, Ph, hexyl) with CH2I2 gave only the resp. syn isomers which were oxidized (no data) to give the resp. ketones. The stereoselective redn. of such trifluoromethyl cyclopropyl ketone derivs. was investigated. E.g. the diastereoselective redn. of cyclopropyl ketone I (R2R3 = O) with L-selectride gave 46% I (R = OH, R1 = H).
~19 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
294. Catalytic phosphorylation of polyfluoro alkanols. 15. Catalytic phosphorylation of α-(polyfluoroalkyl)benzylic alcohols with bis(polyfluoroalkyl) chlorophosphates
By Goryunov, E. I.; Petrovskii, P. V.; Shcherbina, T. M.; Laretina, A. P.; Zakharov, L. S.; Kabachnik, M. I.
From Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya (1990), (4), 878-86. Language: Russian, Database: CAPLUS
Reaction of RC6H4CHR1OH [R = H, m- and p-Me, m-MeO, m-CF3; R1 = CF3, CF2CF2CF3, (CF2)CF3, CF2CF2OCF3] with (R2CH2O)(R3CH2O)P(O)Cl [R2 = R3 = CF3, CF3CF2CF2; R2 = CF3, R3 = H(CF2)6] in the presence of CaCl2 or Mg at 200° gave 82-90% (R2CH2O)(R3CH2O)P(O)(OCHR1C6H4R). The O-alkylation of polyfluorinated alcs. with p- MeC6H4CH(CF3)OP(O)XY (I; X = Y = OCH2CF3, Cl, OPh; X = Cl, Y = OCH2CF3) is a general reaction, and the alkylation ability of I increases in the order of the total electron-accepting effect of substituents X and Y.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
295. Catalytic phosphorylation of polyfluoroalkanols. 14. Synthesis and diastereomeric transformations of aryl (α- polyfluoroalkyl)benzyl chlorophosphates
By Goryunov, E. I.; Petrovskii, P. V.; Zakharov, L. S.; Kabachnik, M. I.
From Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya (1990), (2), 433-41. Language: Russian, Database: CAPLUS
Reaction of m-RC6H4CHR1OH (R = H, CF3, Me; R1 = CF3, CF3CF2CF2, CF3OCF2CF2) with m-R2C6H4OP(O)Cl2 (R2 = H, Me) at 140° in the presence of CaCl2 or Mg gave 62-82% m-RC6H4CHR1OP(O)(Cl)OC6H4R2-m (I; same R-R2) as approx. 50:50 mixts. of diastereomers (thermodn. control). Diastereomeric conversion of I occurred both in soln. (or a melt), or in the solid state.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
296. Unexpected formation of (3,3,3-trifluoropropyl)triphenylphosphonium bromide and subsequent Wittig olefination
By Ullmann, Joerg; Hanack, Michael
From Synthesis (1989), (9), 685-7. Language: English, Database: CAPLUS, DOI:10.1055/s-1989-27357
An unusual rearrangement during the reaction of CF3CHMeBr with PPh3 leads to (3,3,3- trifluoropropyl)triphenylphosphonium bromide (I). Treatment of I with BuLi generates CF3CH2CH:PPh3, which reacts with ketones to give CF3CH2CH:CRR1 (R = R1 = Et, Bu, Ph, CH2Cl; RR1 = (CH2)5, etc.].
~9 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 188 |
297. Catalytic phosphorylation of polyfluoroalkanols. 12. Synthesis and stereochemistry of (1,1-dihydropolyfluoroalkyl) (α-polyfluoroalkylbenzyl) chlorophosphates
By Goryunov, E. I.; Petrovskii, P. V.; Shcherbina, T. M.; Zakharov, L. S.; Kabachnik, M. I.
From Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya (1989), (8), 1853-60. Language: Russian, Database: CAPLUS
Phosphorylation of RC6H4CHR1OH (R = H, 3-Me, 3-CF3, R1 = CF3; R = H, R1 = CF3OCF2CF2) with R3CH2OP(O)Cl2 (R2 = CF3, Bu) in the presence of CaCl2 or Mg at 140° gave 70-76% R2CH2OP(O)ClOCHR1C6H4R as approx. 1:1 mixts. of diastereomers, as obsd. by 1H, 19F, and 31P NMR spectroscopy. Reaction of 4-MeC6H4CH(CF3)OH with CF3CH2OP(O)Cl2 at 90° in the presence of Mg gave a mixt. of 4-MeC6H4CH(CF3)OP(O)ClOCH2CF3, [4- MeC6H4CH(CF3)]2O, and 4-MeC6H4CHClCF3.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
298. Energetic and rate effects of the trifluoromethyl group at C-2 and C-4 on the aliphatic Claisen rearrangement
By Gajewski, Joseph J.; Gee, Kyle R.; Jurayj, J.
From Journal of Organic Chemistry (1990), 55(6), 1813-22. Language: English, Database: CAPLUS, DOI:10.1021/jo00293a027
The rate of the Claisen rearrangement is accelerated by a factor of 73 over the parent system when a trifluoromethyl group is present at C-2 of allyl vinyl ether. Ground-state destabilization by the trifluoromethyl group may be responsible for this rate effect. There is little polar character in the transition state, and the transition-state structure has little carbonyl character and only moderate (ca. 1/3) bonding character between the two terminal carbons. The rate enhancement is not obsd. in the Cope rearrangement of the all-carbon analog that has a trifluoromethyl group at C-2. At C-4, the trifluoromethyl group does not bring about a significant rate effect in the Claisen rearrangement relative to the parent system; this result is in contrast to an energetic benefit of 3.5 kcal/mol enjoyed by the system when a cyano group is at C-4, which suggests that radical-stabilizing ability and not electron-withdrawing ability is important in stabilizing the transition state.
~28 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
299. Alkylnickel and -palladium alkoxides associated with alcohols through hydrogen bonding
By Kim, Yong Joo; Osakada, Kohtaro; Takenaka, Akio; Yamamoto, Akio
From Journal of the American Chemical Society (1990), 112(3), 1096-104. Language: English, Database: CAPLUS, DOI:10.1021/ja00159a032
trans-PdR2L2 (R = Me, Et; L = PMe3, PEt3) and trans-NiMe2(PMe3)2 react with 2 equiv of fluorinated alcs. and parasubstituted phenols to give trans-PdR(OR')(HOR')L2 (R' = CH(CF3)Ph, Ph, p-MeC6H4, p-MeOC6H4, p-ClC6H4, p-BrC6H4, p-FC6H4) and trans-NiMe(OR')(HOR')(PMe3)2 (R' = CH(CF3)Ph, Ph), resp. IR and NMR spectra of these complexes indicate the presence of strong O-H...O hydrogen bonding between the alkoxide (or aryloxide) ligand and the alc. (or substituted and nonsubstituted phenol) both in the solid state and in soln. X-ray crystallog. of transPdMe(OPh)(HOPh)(PMe3)2 and trans-NiMe(OPh)(HOPh)(PMe3)2 shows that the phenoxide oxygen in each complex is assocd. with phenol through hydrogen bonding. Reactions of trans-PdMe2(PMe3)2 with equimolar substituted and nonsubstituted phenols, resp., give trans-PdMe(OC6H4X-p)(PMe3)2 (X = H, Me, OMe, F, Cl, Br), which react with addnl. equimolar phenols to give phenol-bonded palladium complexes trans-PdMe(OC6H4X-p)(HOC6H4X-p)(PMe3)2. transPdMe(OPh)(PMe3)2 also reacts with fluorinated alcs. to give trans-PdMe(OPh)(HOCH(CF3)Ph)(PMe3)2 and transPdMe(OPh)(HOCH(CF3)2)(PMe3)2, which are fully characterized by means of IR and NMR spectroscopy and x-ray crystallog. 1H NMR spectra of mixts. of phenol with cis-PdMe2(dmpe) [dmpe = 1,2-bis(dimethylphosphino)ethane] and cis-PdMe2(dpe) [dpe = 1,2-bis(diphenylphosphino)ethane] indicate formation of strong O-H...O hydrogen bonding
between phenol and the phenoxide ligand in soln. Addn. of phenol to trans-PdMe(OCH(CF3)Ph)(HOCH(CF3)Ph)(PMe3)2 causes displacement of the alkoxide ligand by the phenoxide group to give trans-PdMe(OPh)(HOCH(CF3)Ph)(PMe3)2. Reactions of trans-PdMe(OCH(CF3)Ph)(HOCH(CF3)Ph)(PMe3)2 and trans-PdMe(OPh)(HOCH(CF3)Ph)(PMe3)2 with CO give MeCOOCH(CF3)Ph in 99% and 46% yields, resp. Reactions of trans-PdMe(OCH(CF3)Ph)(HOCH(CF3)Ph)(PMe3)2 with aryl esters give methylpalladium aryloxide complexes and esters of the fluorinated alc. through exchange of the alkoxide group between the complex and the ester. The alkoxide (phenoxide) complexes catalyze transesterification of alcs. with esters. Mechanistic implications of the present results regarding the transesterification are presented.
~120 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 189 |
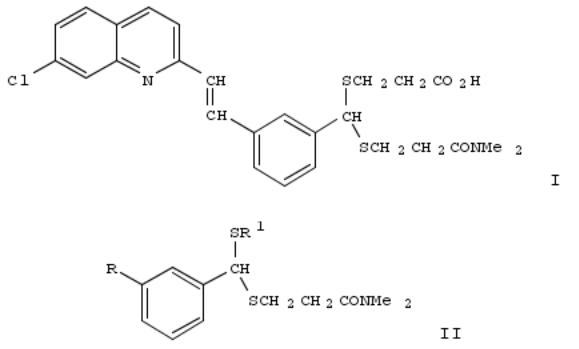
300. Asymmetric dithioacetals. III. The preparation of the enantiomers of 3-((((3-(2-(7-chloroquinolin-2-yl)-(E)- ethenyl)phenyl)-3-dimethylamino-3-oxopropyl-thio)methyl)thio)propionic acid (L-660,711) (MK-571), an antagonist of leukotriene D4
By Young, Robert N.; Gauthier, Jacques Yves; Therien, Michel; Zamboni, Robert
From Heterocycles (1989), 28(2), 967-78. Language: English, Database: CAPLUS
The application of a novel method for the prepn. of chiral dithioacetals to the synthesis of the enantiomers of the title compd. (I) is described. Reaction of 3-Me3CPh2SiOCH2C6H4CHO or isophthalaldehyde with (R)-(-)-MeOCHPhCOSH and HSCH2CH2CONMe2 provides diastereomeric acylthioalkylthioacetals II [R = CHO; Me3Ph2SiCH2; R1 = (R)-(-)- COCHPhOMe] which are readily separable and can be subsequently deacylated with MeONa and the resultant thiolate anion alkylated with Me acrylate to provide enantiomeric dithioacetals II (R = CHO, Me3Ph2SiCH2; R1 = CH2CH2COiMe) which are converted to the enantiomers of I.
~5 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
301. Syntheses of fluoroalkyl and fluoroaryl titanates
By Yoshino, Norio; Tomita, Junko; Hirai, Hidefumi
From Bulletin of the Chemical Society of Japan (1989), 62(7), 2208-12. Language: English, Database: CAPLUS, DOI:10.1246/bcsj.62.2208
Tetrakis(1H,1H-perfluoroethyl) titanate, Ti(OCH2CF3)4, tetrakis(1H,1H-perfluoropropyl) titanate, Ti(OCH2CF2CF3)4, tetrakis(2,2,2-trifluoro-1-methylethyl) titanate, Ti[OCH(CH3)CF3]4, tetrakis(2,2,2-trifluoro-1,1-dimethylethyl) titanate, Ti[OC(CH3)2CF3]4, tetakis((1H,1H,3H-perfluoropropyl) titanate, Ti(OCH2CF2CF2H)4, tetrakis(1H,1H,5H-perfluoropentyl) titanate, Ti[OCH2(CF2CF2)2H]4, tetrakis(1H,1H,7H-perfluoroheptyl) titanate, Ti[OCH2(CF2CF2)3H]4, and tetrakis(1H,1H,9H-perfluorononyl) titanate, Ti[OCH2(CF2CF2)4H]4, were prepd. by the reaction of TiCl4 with fluorosubstituted alcs. in the presence of NH3. Tetrakis(pentafluorophenyl) titanate, Ti(OC6F5)4 was synthesized by the ester interchange reaction of tetraisopropyl titanate with pentafluorophenyl acetate. These compds. were characterized on the basis of chem. anal., IR, 1H NMR, 13C NMR and 19F NMR spectra. Tetrakis(2,2,2-trifluoro-1,1-dimethylethyl) titanate was monomeric in PhNO2, but tetrakis(1H,1H-perfluoroethyl) titanate and tetrakis(1H,1H-perfluoropropyl) titanate were assocd. in the same solvent, in which the highest assocn. nos. obsd. were 3.5 and 1.5, resp. These titanates were very sensitive to moisture and easy to decomp. to give titanium oxide and alc. or phenol.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
302. Optically active biphenylcarboxylic acid esters as liquid crystals and their preparation

SciFinder® |
Page 190 |
By Sato, Masahiro; Yoshio, Kunikyo; Kishiki, Hiroshi; Hoshino, Hiroshi; Nakamura, Toyoichi; Kato, Juji; Naemura, Shohei; Tani, Kazutsuka
From Jpn. Kokai Tokkyo Koho (1989), JP 01139551 A 19890601, Language: Japanese, Database: CAPLUS
The title compds. are described by the general formula RXA1YA2CO2CH(CF3)R1 (I: R, R1 = C1-20 alkyl; X = O, OCO2, single bond; A1, A2 = Q1, Q2, etc.; Q1, Q2, etc., may have substituents; Y = CO2, OCO, C≡C, CH2O, etc.). Reaction of 4- decyloxy-4'-biphenylcarboxylic acid chloride with optically active p-hydroxybenzoic acid 1-trifluoromethylheptyl ester in toluene contg. pyridine gave optically active 4-decyloxy-4'-biphenylcarboxylic acid p'-(1- trifluoromethylheptyloxycarbonyl)phenyl ester.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
303. Asymmetric dithioacetals. II. Novel and versatile method for the preparation of chiral dithioacetals
By Therien, M.; Gauthier, J. Y.; Young, R. N.
From Tetrahedron Letters (1988), 29(51), 6733-6. Language: English, Database: CAPLUS, DOI:10.1016/S0040- 4039(00)82441-7
Reaction of aldehydes with one equiv. each of a thiol and a chiral thioacid such as (R)-(-)-α-methoxyphenylthioacetic acid in the presence of an acidic catalyst such as ZnI2 or p-toluenesulfonic acid yields diastereomeric mixed thioacetals in good yields which are generally readily separable. Subsequent deacylation at low temp. with NaOMe and alkylation of the resulting thiolate anion with a variety of electrophiles provides chiral dithioacetals with no loss of enantiomeric purity.
~7 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
304. Synthetic approach to stereoisomers of allylic alcohols possessing a trifluoromethyl group
By Kitazume, Tomoya; Lin, Jeng Tain; Yamazaki, Takashi
From Journal of Fluorine Chemistry (1989), 43(2), 177-87. Language: English, Database: CAPLUS, DOI:10.1016/S0022-1139(00)82938-3
A no. of stereoisomers of optically pure allylic alcs. with a trifluoromethyl group [CF3CH(OH)CH=CHR; R = Ph, C6H13] were prepd. utilizing the enantiotopic specificity of asym. hydrolysis of their acetates by hydrolases. Their abs. configurations were detd.
~11 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
305. Free-radical approach to carbon-carbon bond formation on a trifluoromethyl-substituted carbon. Intramolecular cyclization
By Morikawa, Tsutomu; Uejima, Masayuki; Kobayashi, Yoshiro
From Chemistry Letters (1989), (4), 623-4. Language: English, Database: CAPLUS, DOI:10.1246/cl.1989.623
A CF3-substituted C radical, generated by radical deoxygenation of the corresponding CF3 carbinol, undergoes intramol. radical cycloaddn. to give a 5- or 6-membered cyclic compd. Thus, treatment of thiocarbamate I with Bu3SnH and AIBN in refluxing C6H6 give 83% of a 1:1 cis-trans mixt. of cyclopentane II.
