
Reference_01_08_2014_165529
.pdf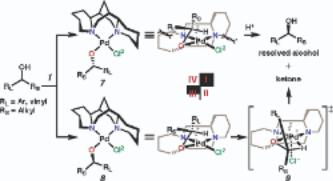
SciFinder® |
Page 161 |
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
228. An Experimentally Derived Model for Stereoselectivity in the Aerobic Oxidative Kinetic Resolution of Secondary Alcohols by (Sparteine)PdCl2
By Trend, Raissa M.; Stoltz, Brian M.
From Journal of the American Chemical Society (2004), 126(14), 4482-4483. Language: English, Database: CAPLUS, DOI:10.1021/ja039551q
A model for asym. induction in palladium-catalyzed aerobic oxidative kinetic resoln. is described. The model is based on coordination complexes and general reactivity trends of the parent (sp)PdCl2 catalyst. The first example of a nonracemic chiral palladium alkoxide complex is presented, and exhibits the subtle steric influences of the C1 sym. ligand sparteine.
~58 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
229. Bookshelf liquid crystal materials and devices
By Wand, Michael; More, Kundalika M.; Chen, Xin-hua; Thurmes, William
From U.S. (2004), US 6703082 B1 20040309, Language: English, Database: CAPLUS
Liq. crystal materials that exhibit bookshelf structures are provided. When these bookshelf materials are incorporated into a bistable host material, the mixt. exhibits a higher switching angle and increased A to C phase transition than bistable hosts without the bookshelf material. These bookshelf materials and bistable compns. are useful in liq. crystal displays, for example.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
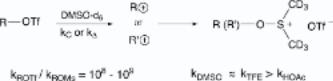
230. Remarkably Facile Solvolyses of Triflates via Carbocationic Processes in Dimethyl Sulfoxide
By Creary, Xavier; Burtch, Elizabeth A.
From Journal of Organic Chemistry (2004), 69(4), 1227-1234. Language: English, Database: CAPLUS, DOI:10.1021/jo0356558
SciFinder® |
Page 162 |
A no. of triflates have been shown to undergo clean pseudo- first-order solvolysis reactions in DMSO-d6 to give products derived from carbocationic intermediates. Thus, t- BuCH(OTf)CO-t-Bu (5) and t-BuCH2OTf (9) react readily in DMSO-d6 at 25 °C to give a rearranged oxosulfonium salts, and subsequent alkene products where Me migration to the incipient cationic center occurs. t-BuCH(OTf)CO2CH3 (14) gives analogous rearranged products, and 1-methylcyclopropyl triflate (21) gives a ring-opened allylic oxosulfonium salt. These triflates react primarily via k pathways. 6- Methylbicyclo[3.1.0]hex-6-yl triflate (23), bicyclo[2.2.1]hept-1-yl triflate (24), 1,6-methano[10]annulen-11-yl triflate (25), (CH3)2C(OTf)CO2CH3 (26), and (CH3)2CCN(OTf) (29) all react in DMSO-d6 to give carbocation-derived products. PhCH(OTf)CF3 (33) and substituted analogs also react readily in DMSO-d6, and the Hammett ρ+ value is -3.7. This suggests a "borderline" mechanism where the transition state has substantial charge development. The primary feature of these solvolyses is the high reactivity of all of these triflates in DMSO- d6. Thus, these triflates are all more reactive in DMSO-d6 than in HOAc, and for most, rates are faster than in CF3CH2OH. Triflates 5, 21, 29, and 33 are 108-109 times more reactive in DMSO-d6 than the corresponding mesylates. It is suggested that the decreased need for electrophilic solvation of triflate anion, and the high cation solvating ability of DMSO, are the reasons for the high triflate reactivity in DMSO-d6.
~11 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
231. Asymmetric synthesis of γ-perfluoroalkyl(aryl) butyrolactones via organoboranes
By Ramachandran, P. Veeraraghavan; Padiya, Kamlesh J.; Rauniyar, Vivek; Reddy, M. Venkat Ram; Brown, Herbert C.
From Tetrahedron Letters (2004), 45(5), 1015-1017. Language: English, Database: CAPLUS, DOI:10.1016/j.tetlet.2003.11.050
Asym. allyl-boration of fluorinated aldehydes with α-pinene-based allyl-boranes provides the corresponding homoallylic alcs. in high ee and de, which upon hydroboration, followed by oxidn. with TPAP/NMO furnish γ-perfluoroalkyl(aryl)-γ- butyrolactones.
~23 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
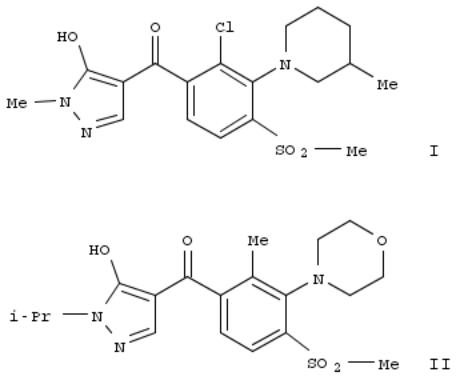
232. The synthesis and biological activity of 1-alkyl-4-(3-azacyclobenzoyl)-5-hydroxypyrazole herbicides
By Benko, Zoltan; Shinkle, Sharon; Van Heertum, John; Jackson, Johnny; McQuiston, Jeffery; Webster, Jeffery; Turner, James; Weimer, Monte; Paterson, Eileen
From Chimia (2003), 57(11), 720-724. Language: English, Database: CAPLUS, DOI:10.2533/000942903777678579
The benzoylpyrazoles belong to a class of herbicides that inhibit the enzyme 4-hydroxyphenylpyruvate dioxygenase (HPPD). This mode of action is characterized by bleaching due to the disruption of plastoquinone and α-tocopherol biosynthesis. Early studies indicated that the C(3) position of benzoylpyrazoles can accommodate a wide range of functionality. This paper describes synthetic efforts to improve cool season grass weed activity and wheat selectivity by incorporating cyclic moieties attached through nitrogen at this position. The aza substituents were generally installed by nucleophilic arom. substitution, however, an efficient four-step method was developed for constructing substituted morpholino moieties directly on pre-formed benzoylpyrazoles. The structure-activity relationships revealed that certain piperidino moieties such as in I provided good activity on wild oat, while exhibiting selectivity toward wheat. They also showed that excellent levels of activity on wild oat and blackgrass can be achieved with morpholino substituents such as in II.
SciFinder® |
Page 163 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
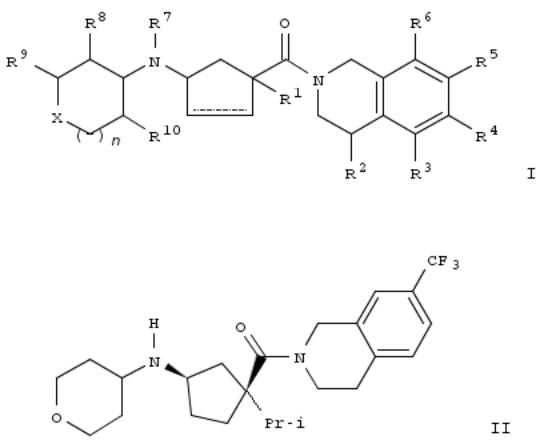
233. Preparation of tetrahydropyranyl cyclopentyl tetrahydroisoquinoline modulators of chemokine receptor activity
By Jiao, Richard; Goble, Stephen D.; Mills, Sander G.; Morriello, Gregori; Pasternak, Alexander; Yang, Lihu; Zhou, Changyou; Butora, Gabor; Kothandaraman, Shankaran; Guiadeen, Deodialsingh; et al
From PCT Int. Appl. (2003), WO 2003093231 A2 20031113, Language: English, Database: CAPLUS
Title compds. I (X = O, S, SO, NH, CR11R12, etc.; R1 = OH, heterocycle, (un)substituted alkyl, alkyloxyalkyl, CN, etc.; R2 = H, OH, halo, heterocycle, (un)substituted alkyl, etc.; R3 = H, OH, halo, CN, NO2, alkylthio, alkylamine, etc.; R4 = F3C, F3CO, H, alkyl, Cl, F, Br, and Ph; R5 = (un)substituted alkyl, alkoxy, alkylthio, pyridyl, F, Cl, Br, heterocycle, etc.; R6 = H, alkyl, F3C, F, Cl, Br; R7 = H, (un)substituted alkyl; R8 = H, F, (un)substituted alkyl, OH, cycloalkyl, etc.; R9 = H, OH, (un)substituted alkyl, carboxylate, alkoxy, or R8 and R9 may be joined together by a alkyl chain or alkyloxyalkyl chain to form a 3-6 membered ring; R10 = H, F, cycloalkyloxy, (un)substituted alkoxy, alkyl, or R8 and R10 may be joined together to form a (un)substituted carbocycle or heterocycle; R11 and R12 = independently H, OH, (un)substituted alkyl, benzyl, alkoxy, etc.; n = 0, 1, and 2) and their pharmaceutically acceptable salts are prepd. and disclosed as modulators of chemokine receptor activity. Thus, II was prepd. by condensation of the precursor aminocyclopentane deriv. (prepn. given) with tetrahydro-4H-pyran-4-one. In particular, these compds. are useful as modulators of the chemokine receptor CCR-2. I had activity in binding to the CCR-2 receptor generally with an IC50 of less than about 1 μM.
SciFinder® |
Page 164 |
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
234. Entrapment of Pseudomonas cepacia lipase with peracetylated β-cyclodextrin in sol-gel: application to the kinetic resolution of secondary alcohols
By Ghanem, Ashraf; Schurig, Volker
From Tetrahedron: Asymmetry (2003), 14(17), 2547-2555. Language: English, Database: CAPLUS, DOI:10.1016/S0957-4166(03)00550-0
Co-lyophilized Pseudomonas cepacia lipase with peracetylated β-cyclodextrin was immobilized by the sol-gel process. The gel-entrapped lipase/cyclodextrin was prepd. by the hydrolysis of methyltrimethoxysilane (MTMS) in the presence of the co-lyophilized lipase with peracetylated β-cyclodextrin prepd. with different wt. ratios (enzyme to CD). This type of enzyme prepn. was subsequently used in the kinetic resoln. of a set of secondary alcs. using isopropenyl acetate as an innocuous acyl donor in toluene as the org. medium. The resulting chiral alcs. (substrate) and the corresponding acetates (product) were baseline sepd. in one anal. without derivatization using gas chromatog. on a new chiral stationary phase (CSP) Chirasil-β-Dex contg. an undecamethylene spacer (C11-Chirasil-Dex).
~19 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
235. Generation of an optically active rhodium(III) complex by crystallization-induced spontaneous resolution of a racemic mixture
By Odinets, Irina L.; Artyushin, Oleg I.; Sharova, Elena V.; Goryunov, Eugenii I.; Golovanov, Denis G.; Lyssenko, Konstantin A.; Petrovskii, Pavel V.; Mastryukova, Tatyana A.
From Mendeleev Communications (2003), (3), 102-104. Language: English, Database: CAPLUS, DOI:10.1070/MC2003v013n03ABEH001778
Racemic (π-pentamethylcyclopentadienyl)(2,2,2-trifluoro-1-phenylethyl diethylphosphinite)rhodium dichloride (2) was found to be a conglomerate, which undergoes spontaneous resoln. during crystn. Complex 2 was prepd. in 78% yield via reaction of 2,2,2-trifluoro-1-phenylethyl diethylphosphinite (1) and [Cp*RhCl2]2.
SciFinder® |
Page 165 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
236. A highly efficient room temperature non-organometallic route for the synthesis of α,β,β-trifluorostyrenes by dehydrohalogenation
By Anilkumar, R.; Burton, Donald J.
From Tetrahedron Letters (2003), 44(35), 6661-6664. Language: English, Database: CAPLUS, DOI:10.1016/S0040- 4039(03)01628-9
Various 1-aryl-1,2,2,2-tetrafluoroethanes were synthesized by the fluorination of the corresponding alcs. with DAST. Dehydrofluorination of ArCHFCF3 using lithium hexamethyldisilazide (LHMDS) base in THF at room temp. produced 1,2,2-trifluorostyrenes (ArCF=CF2) in 61-91% isolated yields. This procedure provides an excellent non-organometallic alternative to the generally used metalation-Pd(0) coupling methods. For example, redn. of 2,2,2-trifluoro-1- phenylethanone gave α-(trifluoromethyl)benzenemethanol. Fluorination of this using DAST gave (1,2,2,2- tetrafluoroethyl)benzene which was dehydroflorinated using 1,1,1-trimethyl-N-(trimethylsilyl)silanamine lithium salt (LHMDS) to give (1,2,2-trifluoroethenyl)benzene (I). The dehydrochlorination of (2-Chloro-1,2,2-trifluoroethyl)benzene gave also I in 94% using LHMDS.
~14 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
237. Lipase-catalyzed Irreversible Transesterification of Secondary Alcohols Using Isopropenyl Acetate
By Ghanem, Ashraf
From Monatshefte fuer Chemie (2003), 134(8), 1151-1157. Language: English, Database: CAPLUS, DOI:10.1007/s00706-003-0025-1
Asym. acetylation of a set of secondary alcs. with the innocuous acyl donor isopropenyl acetate catalyzed by a lipase from Pseudomonas cepacia immobilized on ceramic particles (PSL-C) in toluene as org. medium afforded the chiral alcs. and the corresponding acetates in high enantiomeric excess (up to 99%). An effective baseline sepn. of the enantiomers of both substrate and product was performed in one anal. without derivatization using gas chromatog. on a new chiral stationary phase (CSP) Chirasil-β-Dex contg. an undecamethylene spacer (C11-Chirasil-Dex).
~10 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
238. The polar effect on the regiochemistry of nucleophilic substitution of trifluoromethylated π-allylpalladium complex
By Okano, Takashi; Matsubara, Hiroyoshi; Kusukawa, Takahiro; Fujita, Makoto
From Journal of Organometallic Chemistry (2003), 676(1-2), 43-48. Language: English, Database: CAPLUS, DOI:10.1016/S0022-328X(03)00262-6
Allylic nucleophilic substitution of trifluoromethyl-substituted cinnamyl carbonate with di-Et malonate anion in the presence of palladium complex catalyst gave regioand stereoselectively the more sterically crowded SN2' product. 1- Trifluoromethylcinnamyl Et carbonates RCH:CHCH(CF3)OCO2Et (1a-f, R = Ph, 2-MeC6H4, 2,4,6-Me3C6H2, tBu, 2- MeOC6H4, 2-MeOCH2OC6H4) were prepd. in two steps by redn. of the corresponding ketones and esterification by Et chloroformate. Esters 1a-f were reacted with CH2(CO2Me)2 in the presence of NaOEt and [(allyl)PdCl]2-PPh3 catalyst, giving exclusively di-Me (1-R-4,4,4-trifluoro-2-butenyl)malonate regioisomers (5a-f). The regiochem. caused by the polar effect of trifluoromethyl group was opposite to the methylated cinnamyl substrate in a similar steric environment. The sterically more hindered mesityl and tert-Bu substrates than Ph deriv. also gave the products reacted at the more hindered sites. Although o-substituted substrates expecting intramol. coordination to affect regiochem. were examd., no alternative regioisomers were detected.
~17 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
239. Scope of Enantioselective Palladium(II)-Catalyzed Aerobic Alcohol Oxidations with (-)-Sparteine
By Mandal, Sunil K.; Jensen, David R.; Pugsley, Jacob S.; Sigman, Matthew S.
From Journal of Organic Chemistry (2003), 68(11), 4600-4603. Language: English, Database: CAPLUS, DOI:10.1021/jo0269161
SciFinder® |
Page 166 |
Evaluation of the substrate scope for Pd(II)/(-)-sparteine catalyzed aerobic oxidative kinetic resoln. of secondary alcs. is disclosed. An improved system is found with use of Me3COH solvent in which benzylic and aliph. alcs. as well as alcs. contg. olefins are effectively oxidatively resolved. For substrates that successfully undergo oxidative kinetic resoln., krel values are generally between 10 and 20. Successful scale-up of various substrates to 10-mmol scale is described.
Extension to oxidative desymmetrization of 1,3-meso-diols is successful with enantiomeric excesses ranging from 78 to 85%.
~70 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
240. New Silyl Ether Reagents for the Absolute Stereochemical Determination of Secondary Alcohols
By Williamson, R. Thomas; Sosa, Ana C. Barrios; Mitra, Abhijit; Seaton, Pamela J.; Weibel, Douglas B.; Schroeder, Frank C.; Meinwald, Jerrold; Koehn, Frank E.
From Organic Letters (2003), 5(10), 1745-1748. Language: English, Database: CAPLUS, DOI:10.1021/ol034418o
Nonracemic silanes (R)-PhCH(CF3)OSiMe2Cl (I) and (S)-PhCH(CF3)OSiMe2Cl (II) are prepd. as anal. reagents for the detn. of the enantiomeric purity and abs. stereochem. of secondary alcs. Treatment of secondary alcs. sep. with I and II allows for the detection and quantitation of enantiomers in a sample. The differences in the NMR shifts of protons between the (R)-α-trifluoromethylbenzyl silyl ether and the (S)-α-trifluoromethylbenzyl silyl ether correlate with the abs. configuration at the secondary alc. stereocenter; the correlation holds for a diverse set of secondary alcs. such as 2- butanol, (R)-pantolactone, podophyllotoxin, cholesterol, and cytochalasin B. Deprotection of the silyl ethers with tetrabutylammonium fluoride on silica gel yields the initial alcs. (no data). The conformational preferences of the silyl ether derived from reaction of II with 4-heptanol are detd. by Monte Carlo calcns.
~16 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
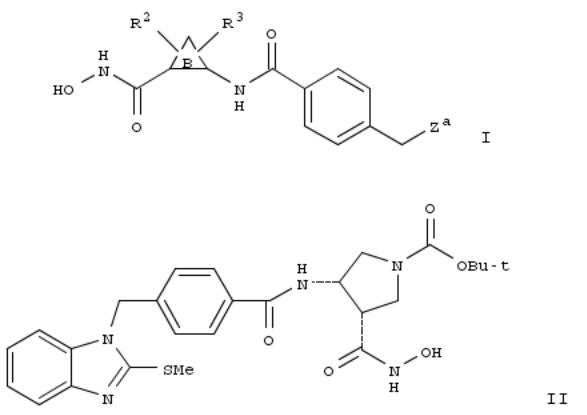
241. Preparation of cyclic hydroxamic acids as inhibitors of matrix metalloproteinases and/or TNF-α converting enzyme for treatment of inflammatory disorders
By Ott, Gregory; Chen, Xiao-Tao; Duan, Jingwu; Lu, Zhonghui
From PCT Int. Appl. (2003), WO 2003024899 A2 20030327, Language: English, Database: CAPLUS
Title compds. I [wherein ring B = (un)substituted 4-7 membered (hetero)cyclic ring contg. 0-2 O, N, NR1, or SOp atoms and 0-3 carbonyl groups; R1 and R2 = independently Q, alk(en/yn)ylene-Q, or (un)substituted alkylene-Q interrupted by O, NRa, CO, CO2, CONRa, NRaCO, NRaCO2, NRaCONRa, SOp, NRaSO2, or SO2NRa; or R1 = (un)substituted alkylene-Q interrupted by OCO, OCO2, or OCONRa; Q = H or (un)substituted (hetero)cyclyl; R3 = Q1, Cl, F, alk(en/yn)ylene-Q1, or (un)substituted alkylene-Q1 interrupted by O, NR1, NRaCO, CONRa, CO, CO2, SOp, or SO2NRa; Q1 = H or (un)substituted Ph, naphthyl, or heterocyclyl; Za = (un)substituted benzimidazolyl, indolyl, imidazopyridinyl, pyrazolylpyridinyl, benzofuranyl, benzothiazinyl, quinolinyl, etc.; Ra = independently H, alkyl, Ph, or benzyl; p = 0-2; or stereoisomers or pharmaceutically acceptable salts thereof] were prepd. as inhibitors of matrix metalloproteinases (MMP), TNF-a converting enzyme (TACE), aggrecanase, or a combination thereof. For example, reaction of benzyl Me maleate with paraformaldehyde and glycine gave benzyl Me (cis)-3,4-pyrrolidinedicarboxlyate (100%). BOC-protection (64%), debenzylation (96%), resoln. of the (3S,4S)-isomer with (S)-α-methylbenzylamine, conversion to the carbamate with DPPA and PhCH2OH (76%), and Pd catalyzed hydrogenation (100%) provided Me (3S,4S)-4-amino-1-(tert- butoxycarbonyl)-3-pyrrolidinecarboxylate. Coupling of the amine with 4-[(2-methylthio-1H-benzimidazol-1- yl)methyl]benzoic acid (prepn. given) afforded the amide (99%), which was treated with NH2OH•HCl/MeONa to give the hydroxamic acid (3S,4S)-II (33%). A no. of the compds. of the invention inhibited MMP-1, 2, 3, 7, 8, 9, 10, 12, 13, 14, 15, and/or 16 with Ki values of ≤ 10 μM. Thus, I are useful for the treatment of a wide variety of inflammatory disorders (no data).
SciFinder® |
Page 167 |
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
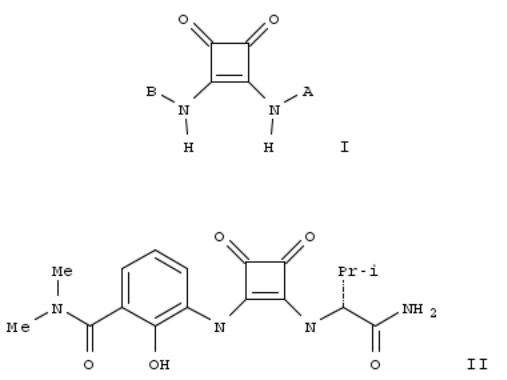
242. Preparation of 3,4-di-substituted cyclobutene-1,2-diones as cxc-chemokine receptor ligands
By Taveras, Arthur G.; Aki, Cynthia J.; Bond, Richard W.; Chao, Jianping; Dwyer, Michael; Ferreira, Johan A.; Chao, Jianhua; Yu, Younong; Baldwin, John J.; Kaiser, Bernd; et al
From PCT Int. Appl. (2002), WO 2002083624 A1 20021024, Language: English, Database: CAPLUS
Title compds. I [A = (un)substituted heterocycle, heterocyclealkyl, heteroaryl, heteroarylalkyl, cycloalkyl, etc.; B = (un)substituted aryl, heteroaryl, heterocycle, heteroarylarene, etc.], or a pharmaceutically acceptable salt or solvate thereof, are prepd. and disclosed as cxc-chemokine receptor ligands. Thus, II was prepd. by substitution of (dimethylaminocarbonylhydroxyphenylamino)(ethoxy)cyclobutenedione [prepn. given] with (R)-2-amino-N,3- dimethylbutanamide monohydrochloride [prepn. given]. Compds. of the invention demonstrated an IC50 value of < 20 μM in CXCR1 SPA assay and < 5 μM in CXCR2 SPA assay. I are useful for the treatment of chemokine-mediated diseases such as acute and chronic inflammatory disorders and cancer.
SciFinder® |
Page 168 |
~20 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
243. Facile oxidation of alcohols to carbonyl compounds using a tris(2-methylphenyl) bismuth dichloride-DBU binary system
By Matano, Yoshihiro; Nomura, Hazumi
From Angewandte Chemie, International Edition (2002), 41(16), 3028-3031. Language: English, Database: CAPLUS, DOI:10.1002/1521-3773(20020816)41:16<3028::AID-ANIE3028>3.0.CO;2-U
High efficiency and chemoselectivity as well as the facile isolation of the carbonyl products by simple workup procedures characterize a new method of alc. oxidn. A variety of primary and secondary alcs. are oxidized to aldehydes and ketones by the combined use of tris(2-methylphenyl)bismuth dichloride (I) and 1,8-diazabicyclo[5,4,0]undec-7-ene (DBU) under mild conditions. The oxidn. of 4-bromobenzenemethanol gave 4-bromobenzaldehyde in 94% yield in the presence of I and DBU. Chemoselective oxidn. of 4-phenyl-3-buten-2-ol in the presence of I and DBU gave 4-phenyl-3-buten-2-one in >99% yield.
~12 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
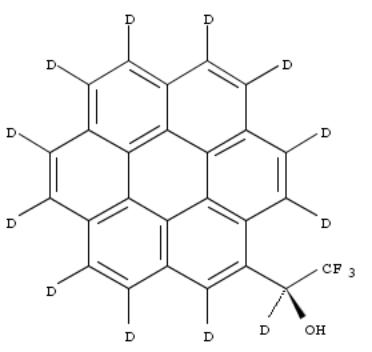
244. Preparation of homochiral aromatic compounds as chiral solvating agents and chiral auxiliaries
By Virgili Moya, Albert
From Span. (2001), ES 2160464 A1 20011101, Language: Spanish, Database: CAPLUS
Homochiral compds. X-A-CR1R2R3 [A represents a deuterated arom. or heteroarom. ring system (1-6 rings); X = D, halo,
an electrophilic group such as NO2 or an electron donor group such as OR' or NR'R'', where R' and R'' = alkyl, aryl, acyl, D or H; R1 = H or D; R2 = OR', NR'R'''; R3 = CF3, alkyl, aryl, or acyl which is optionally deuterated or fluorinated] were prepd. for use as chiral solvating agents and chiral auxiliaries. These compds. do not produce a signal in 1H NMR.
Thus, (R)-perdeutero-2,2,2-trifluoro-1-(9-anthryl)ethanol (I) was prepd. from perdeuterated anthracene by trifluoroacetylation, redn. with LiAlD4, acetylation, resoln. by HPLC, and sapon. The 1H NMR of (±)-1-phenyl-1,2- ethanediol carried out at 400 MHz in the presence of I showed distinct signal for each enantiomer.
SciFinder® |
Page 169 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
245. Synthesis of trifluoromethyl ketones using polymer-supported reagents
By Baxendale, I. R.; Ley, S. V.; Lumeras, W.; Nesi, M.
From Combinatorial Chemistry and High Throughput Screening (2002), 5(3), 197-199. Language: English, Database: CAPLUS, DOI:10.2174/1386207024607220
A two step synthesis of trifluoromethyl ketones from aldehydes is reported. A combination of polymer-supported reagents and sequestering agents were employed to effect the transformation without the need for chromatog. purifn. For example, trifluoromethylation of 4-methoxybenzaldehyde with (trifluoromethyl)trimethylsilane in the presence of Amberlyst-27 (fluoride form), followed by treatment with Amberlyst-15 and AM-resin gave 4-methoxy-α- (trifluoromethyl)benzenemethanol in 100% yield. Oxidn. of the latter using Amberlyst-27-supported permanganate (MnO4 1-) gave 2,2,2-trifluoro-1-(4-methoxyphenyl)ethanone (100% yield). Under these conditions only aryl alcs. cleanly afforded the corresponding trifluoromethyl ketones.
~13 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
246. The effect of catechin derivatives on the enantioselectivity of lipase-catalyzed hydrolyses of alkynol benzoate esters
By Nakamura, Kaoru; Takenaka, Keishi
From Tetrahedron: Asymmetry (2002), 13(4), 415-422. Language: English, Database: CAPLUS, DOI:10.1016/S0957- 4166(02)00121-0
Polyphenols, such as (+)-catechin and pyrogallol, enhance stereochem. control in the lipase-catalyzed hydrolysis of alkynol benzoate esters PhCO2CHR1C≡CR2 (R1 = Me, Et, Pr, Bu, n-pentyl; R2 = H, Me, Et) and benzyl benzoates PhCO2CHPhR3 (R3 = Me, F3C), leading to increased enantioselectivities in the kinetic resoln. of alkynols and benzyl alcs. with lipase Amano AH.
~16 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
247. Partially fluorinated liquid crystal material
By Wand, Michael; Gough, Neil; Chen, Xin Hua
From PCT Int. Appl. (2002), WO 2002018514 A1 20020307, Language: English, Database: CAPLUS
SciFinder® |
Page 170 |
The invention provides LC compns. that exhibit V-shaped switching when aligned in an analog device configuration and exhibit bistable switching when aligned in a bookshelf-type device configuration. The invention more specifically provides LC compns. of (R = fluorinated alkyl, ether; A, B, C = 5-6 arom. rings each substituted with 1-4 fluorines and CH can be substituted with N, O, S; d = 0, 1; D = COO, OOC, CH2CH2, double bond, triple bond; Y = C1-6 alkyl, fluorinated alkyl; R1 = nonchiral tail alkyl with CH2 group replaced by O, S, etc.) which exhibit bistable switching as well as V-shaped switching when aligned in appropriate device configurations. The invention also provides methods of using the compds. of the invention in making LC compns. and electrooptical devices comprising an aligned layer of the compns. of this invention.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
248. Concise Syntheses of Nonracemic γ-Fluoroalkylated Allylic Alcohols and Amines Via an Enantiospecific PalladiumCatalyzed Allylic Substitution Reaction
By Konno, Tsutomu; Nagata, Kensuke; Ishihara, Takashi; Yamanaka, Hiroki
From Journal of Organic Chemistry (2002), 67(6), 1768-1775. Language: English, Database: CAPLUS, DOI:10.1021/jo011013d
α-Fluoroalkylated allyl mesylates reacted with various carboxylates and amines in the presence of tetrakis(triphenylphosphine)palladium(0) catalyst to give the corresponding γ-fluoroalkylated (E)-allylic alc. derivs. and amines, resp., in excellent yields. In almost all cases, no other regioand stereoisomers were produced. Application of this palladium-catalyzed allylic substitution reaction to various nonracemic mesylates afforded chiral γ-fluoroalkylated allylic alc. derivs. and amines without any loss of enantiomeric excess through the reaction.
~36 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
249. Preparation of O-substituted 4-(4-hydroxyphenyl)-1,1,1-trifluoro-2-butanones as selective cPLA2 inhibitors
By Banville, Jacques; Gai, Yonghua; Johnson, Graham; Zusi, Fred Christopher; Burke, James R. From U.S. (2002), US 6350892 B1 20020226, Language: English, Database: CAPLUS
Title compds. I [R5 = alk(en/yn)yl, alkoxy, alkylthio, halo, hydroxy, etc.; p = 0-2; V1 = O, S0-2, NHC:O, C:ONH; R3-4 = H, Me; R1-2 = when taken together form an oxo group or R1-2 = H, OH; Y1 = O, S0-2, aza, etc.] were prepd. E.g., 4-(3- hydroxypropyl)phenol was converted to Me [4-(3-methanesulfonyloxypropyl)phenoxy]acetate in 4 steps. This intermediate was reacted with N-methyl-2,2-[di(4-chlorophenyl)]ethylamine (CH3CN, NaI, 80°C, 18 h) to give the corresponding tertiary amine. The amine was treated with trifluoromethyltrimethylsilane (PhMe, -55°C) to give isolated acetal II. Hydrolysis of II (THF, HClaq) provided the example compd. trifluoromethylketone isolated as the hydrochloride. Compds. I, presented in examples, showed IC50 of 1-50 μM against cPLA2.
