
Reference_01_08_2014_165529
.pdf
SciFinder® |
Page 81 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
105. Production of methyl fluoroalkyl ethers by reaction of chloromethane with fluoroalkoxides
By Kovalenko, Serguei V.; Swinson, Joel
From U.S. Pat. Appl. Publ. (2010), US 20100312019 A1 20101209, Language: English, Database: CAPLUS
A process for the prepn. of a Me fluoroalkyl ether comprises reacting an alkoxide of a fluoroalkyl alc. with chloromethane, wherein the alkoxide is selected from sodium and potassium alkoxides of 2,2,2-trifluoroethanol, 1,1,1,3,3,3- hexafluoroisopropanol and 1,1,1-trifluoroisopropanol. The unreacted chloromethane may be recovered and recycled in a relatively simple way, thus reducing process cost and environmental problems assocd. with the use of di-Me sulfate.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
106. Fluorine-containing 1,6-diene ether compound and fluorine-containing polymer
By Kubota, Toshio; Yanai, Ko-Ichi; Nagasawa, Takehiro; Hirooka, Akira
From PCT Int. Appl. (2010), WO 2010134509 A1 20101125, Language: Japanese, Database: CAPLUS
A fluorine-contg. polymer obtained by using 1,6-diene ether compd. I [R = (substituted) C1-12 alkyl, C6-18 aryl] is a highperformance polymer exhibiting a low refractive index, a high glass transition point, a high degree of transparency, and soly. in solvents. There are many potential uses, such as use as a coating material or bulk material. For example, said polymer could be effectively used in high-tech fields such as: optical materials such as low-reflection films and optical waveguide cladding; semiconductor materials such as pellicles and resists in semiconductor lithog.; and protective film materials, insulating film materials, and water-repellent materials.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
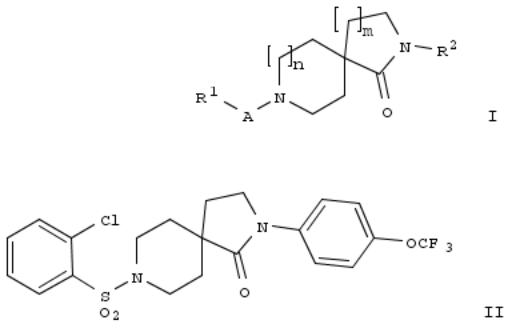
107. Preparation of azacyclic spiro derivatives as HSL inhibitors
SciFinder® |
Page 82 |
By Ackermann, Jean; Conte, Aurelia; Hunziker, Daniel; Neidhart, Werner; Nettekoven, Matthias; Schulz-Gasch, Tanja; Wertheimer, Stanley
From PCT Int. Appl. (2010), WO 2010130665 A1 20101118, Language: English, Database: CAPLUS
The title compds. I [m = 1-2; n = 0-2 (when n = 0 then m = 1); A = SO2 or C(O); R1 = alkyl, cycloalkyl, aminoalkyl, etc.; R2 = imidazolyl, Ph, pyridinyl, etc.], useful as HSL inhibitors for the treatment of diabetes, dyslipidemia, atherosclerosis and obesity, were prepd. and formulated. E.g., a multi-step synthesis of II, starting from piperidine-1,4-dicarboxylic acid 1- tert-Bu ester 4-Et ester and 1-bromo-2-methoxyethane, was given. Exemplified compds. I were tested against human hormone-sensitive lipase (HSL). For example, II showed IC50 of 0.03 μM.
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
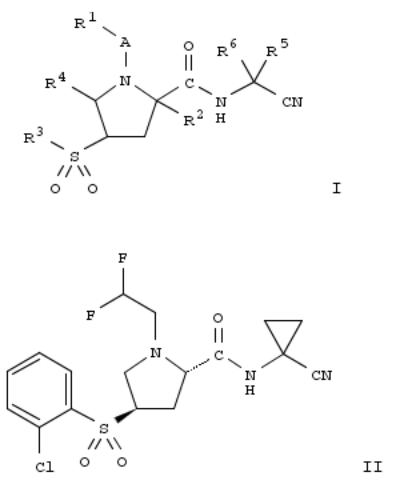
108. Preparation of proline dipeptidyl nitrile derivatives as cathepsin, particularly cathepsin S and L, inhibitors
By Sanchez, Ruben Alvarez; Banner, David; Ceccarelli, Simona M.; Grether, Uwe; Haap, Wolfgang; Hartman, Peter; Hartmann, Guido; Hilpert, Hans; Kuehne, Holger; Mauser, Harald; et al
From U.S. Pat. Appl. Publ. (2010), US 20100267722 A1 20101021, Language: English, Database: CAPLUS
The invention is related to the prepn. of title compds. I [R1 = H, alkyl, alkoxy, alkoxycarbonylaminocycloalkyl, oxetanyl, etc.; A = absent, CH2, CH2CH2, CO, COO, SO2; R2 = H, haloalkyl, cycloalkyl, phenylsulfonylalkyl, etc.; or A, R1 and R2 together form CH2CH2, CH2CF2CH2, CH2CH2CH2, CH2CH2CH2CH2; CH2CH2OCH2, CH2CH2CH(C80N); R3 = hydroxyalkyl, Ph, alkyl, etc.; R4 = H, alkylphenyl, cycloalkyloxy, etc.; R5, R6 = H, Ph, haloalkyloxy, etc.; or CR5R6 = cycloalkyl, pyrrolidinyl or piperidinyl] and their pharmaceutically acceptable salts as inhibitors of cysteine protease cathepsin, in particular of cathepsin S and L. Thus, a multi-step synthesis from 1-tert-Bu 2-Me (2S,4R)-4- hydroxypyrrolidine-1,2-dicarboxylilate was given for II. I inhibited cathepsin S with IC50 values in the range of 0.00001 and 100 μM and cathepsin L with IC50 values in the range of 0.00001 and 200 μM. Pharmaceutically compns. contg. I are also described.
SciFinder® |
Page 83 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
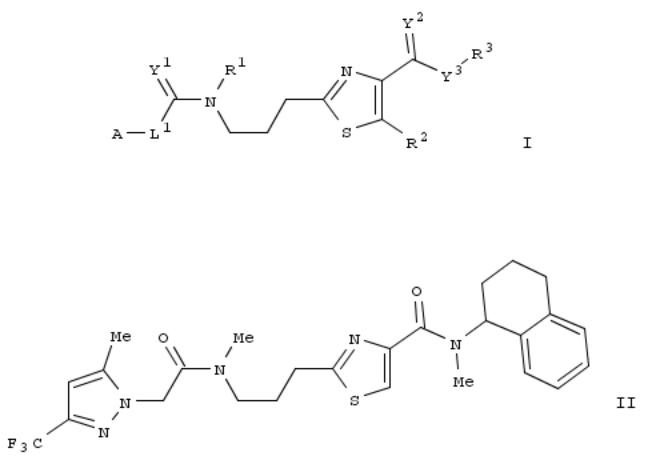
109. Aminopropyl thiazole derivatives useful as fungicides, and their preparation procedures
By Cristau, Pierre; Rahn, Nicola; Tsuchiya, Tomoki; Herrmann, Stefan; Wachendorff-Neumann, Ulrike; Benting, Juergen
From Ger. Offen. (2010), DE 102010000662 A1 20101021, Language: German, Database: CAPLUS
The invention relates to aminopropyl thiazole derivs. of formula I, wherein the symbols A, L1, L2, Y1, Y2, Y3, R1, R2, R3 have the meanings indicated in the description, agrochem. effective salts thereof, use thereof to fight against plantpathogenic harmful fungi as well as procedures for the prepn. thereof. In an example, compd. II was prepd. by multistep synthesis. From the std. antifungal assays, compd. II was found to inhibit Phytophophora in tomato test (³70% at 500 ppm) and Plasmopara in vine test (³70% at 100 ppm).
SciFinder® |
Page 84 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
110. Design, synthesis, and subtype selectivity of 3,6-disubstituted β-carbolines at Bz/GABA(A)ergic receptors. SAR and studies directed toward agents for treatment of alcohol abuse
By Yin, Wenyuan; Majumder, Samarpan; Clayton, Terry; Petrou, Steven; VanLinn, Michael L.; Namjoshi, Ojas A.; Ma, Chunrong; Cromer, Brett A.; Roth, Bryan L.; Platt, Donna M.; et al
From Bioorganic & Medicinal Chemistry (2010), 18(21), 7548-7564. Language: English, Database: CAPLUS, DOI:10.1016/j.bmc.2010.08.049
A series of 3,6-disubstituted β-carbolines was synthesized and evaluated for their in vitro affinities at αxβ3γ2 GABAA/benzodiazepine receptor subtypes by radioligand binding assays in search of α1 subtype selective ligands to treat alc. abuse. Analogs of β-carboline-3-carboxylate-t-Bu ester (βCCt, 1) were synthesized via a CDI-mediated process and the related 6-substituted β-carboline-3-carboxylates 6 including WYS8 (7) were synthesized via a Sonogashira or Stille coupling processes from 6-iodo-βCCt (5). The bivalent ligands of βCCt (32 and 33) were also designed and prepd. via a palladium-catalyzed homocoupling process to expand the structure-activity relationships (SAR) to larger ligands. Based on the pharmacophore/receptor model, a preliminary SAR study on 34 analogs illustrated that large substituents at position-6 of the β-carbolines were well tolerated. As expected, these groups are proposed to project into the extracellular domain (LDi region) of GABAA/Bz receptors (see 32 and 33). Moreover, substituents located at position-3 of the β-carboline nucleus exhibited a conserved stereo interaction in lipophilic pocket L1, while N(2) presumably underwent a hydrogen bonding interaction with H1. Three novel β-carboline ligands (βCCt, 3PBC and WYS8), which preferentially bound to α1 BzR subtypes permitted a comparison of the pharmacol. efficacies with a range of classical BzR antagonists (flumazenil, ZK93426) from several different structural groups and indicated these β-carbolines were near GABA neutral antagonists'. Based on the SAR, the most potent (in vitro) α1 selective ligand was the 6-substituted acetylenyl βCCt (WYS8, 7). Earlier both βCCt and 3PBC had been shown to reduce alc. self-administration in alc. preferring (P) and high alc. drinking (HAD) rats but had little or no effect on sucrose self-administration. Moreover, these two β-carbolines were orally active, and in addn., were anxiolytic in P rats but were only weakly anxiolytic in rodents. These data prompted the synthesis of the β-carbolines presented here.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.

SciFinder® |
Page 85 |
111. Preparation of indole derivatives and methods for antiviral treatment
By Maccoss, Malcolm; Njoroge, F. George; Nomeir, Amin; Chen, Guangming; Huang, Song Xiao; Kakarla, Ramesh; Karp, Gary Mitchell; Lennox, William Joseph; Li, Chunshi; Liu, Ronggang; et al
From PCT Int. Appl. (2010), WO 2010117932 A1 20101014, Language: English, Database: CAPLUS
The invention is directed to compds. of formula I and forms and pharmaceutical compns. thereof useful for treating a viral infection, or for affecting viral activity by modulating viral replication. Compds. of formula I wherein X is H, halo, CN, NO2, etc.; Ar is (un)substituted heteroaryl and (un)substituted heterocyclyl; Z is C1-8 alkyl, C2-8 alkenyl-C1-8 alkyl, C2-8 alkenyl-C1-8 alkyl, etc.; R1 is aminosulfonylalkyl, aminosulfonylamino, sulfonylamino, etc.; R2 is 1 - 3 substituents each is independently H, halo, OH, CN, NO2, etc.; and free acids, free bases, salts, hydrates, solvates, clathrates, isotopologues, racemates, enantiomers, diastereoisomers, and polymorphs thereof, are claimed. Example compd. II was prepd. by a general procedure procedure given. All the invention compds. were evaluated for their antiviral activity. From the assay, it was detd. that compd. II exhibited an IC50 value of less than about 0.5 μM.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
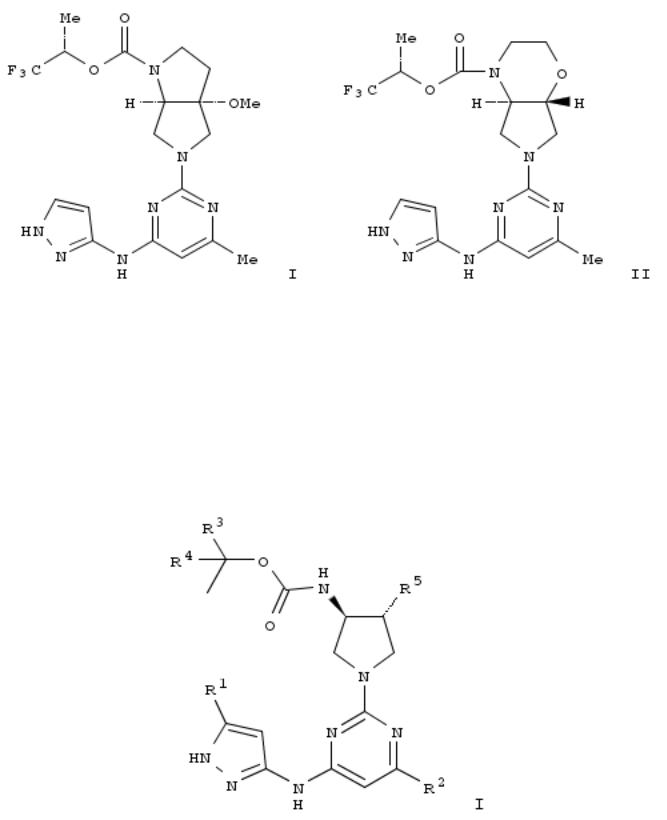
112. Preparation of pyrazolylaminopyrimidinyl pyrrolopyrrolecarboxylates and pyrrolooxazinecarboxylates as aurora A selective inhibitors
By Iwama, Toshiharu; Kawanishi, Nobuhiko; Mortimore, Michael; Ohkubo, Mitsuru; Sunami, Tomoko From PCT Int. Appl. (2010), WO 2010111057 A1 20100930, Language: English, Database: CAPLUS
The title compds. such as I and II are prepd. I is prepd. starting from tert-Bu (3S)-3-(tert-butyldimethylsilyl)oxy-4- oxopyrrolidine-1-carboxylate.
SciFinder® |
Page 86 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
113. Preparation of pyrazolylaminopyrrolidinylpyrimidines as Aurora A kinase inhibitors.
By Binch, Hayley; Hashimoto, Masaya; Iwama, Toshiharu; Kawanishi, Nobuhiko; Mortimore, Michael; Ohkubo, Mitsuru; Sunami, Tomoko
From PCT Int. Appl. (2010), WO 2010111050 A1 20100930, Language: English, Database: CAPLUS
Title compds. [I; R1, R2 = H, alkyl; R3, R4 = H, (substituted) alkyl; R5 = H, OH, alkyl, OMe], were prepd. Thus, (2S)-1,1,1- trifluoropropan-2-yl [(3S)-1-[4-methyl-6-(1H-pyrazol-3-ylamino)pyrimidin-2-yl]pyrrolidin-3-yl]carbamate [4-step prepn. from
(S)-1,1,1-trifluoro-2-propanol, (3S)-1-benzylpyrrolidin-3-amine, and 2-chloro-6-methyl-N-(1H-pyrazol-3-yl)pyrimidin-4- amine given] inhibited Aurora A kinase with IC50 = 3.6 nM.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
114. A study on the kinetic resolution of benzyl alcohols bearing functional group on α-position catalyzed by (R)- benzotetramisole catalyst
SciFinder® |
Page 87 |
By Chen, Peiran; Zhang, Yi; Zhou, Hui; Xu, Qing
From Huaxue Xuebao (2010), 68(14), 1431-1436. Language: Chinese, Database: CAPLUS
Kinetic resoln. of benzyl alcs. bearing functional group on α-position catalyzed by (R)-benzotetramisole catalyst were studied. The competition between the cation-π interaction of the aryl ring of the substrates with the catalyst and the cation-π interaction of the functional group's π-system with the catalyst decreased the stereo-selectivity of the reaction and the configuration change of the products.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
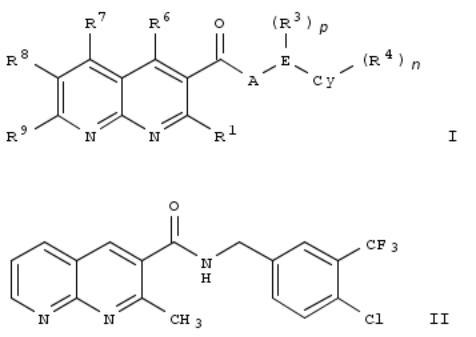
115. 1,8-Naphthyridine derivatives as bronchodilators and their preparation and use for the treatment of respiratory diseases
By Johansson, Martin; Thornqvist-Oltner, Viveca; Toftered, Joergen; Wensbo, David; Dalence, Maria From PCT Int. Appl. (2010), WO 2010097410 A1 20100902, Language: English, Database: CAPLUS
The invention relates to 1,8-naphthyridine derivs. of formula I, which are bronchodilators and which are useful in the treatment of respiratory diseases. Compds. of formula I wherein R1 is C1-3 alkyl, NH2 and derivs., C1-3 fluoroalkyl, etc.; A is NH2 and derivs., O and S; E is C1-3 alkylene, ethene-1,2-diyl, 1-propene-1,3-diyl and 2-propene-1,3-diyl; each R3 is independently C1-3 alkyl, OMe, C1-5 fluoroalkyl, etc.; p is o to 2; Cy is 5- to 6-membered heteroaryl and phenyl; each R4 is independently C1-8 alkyl, C1-5 fluoroalkyl, halo, etc.; n is 0 to 2; R6, R7 and R8 are independently H, halo, C1-3 alkyl, NHC0-3 alkyl, etc.; R9 is H, halo, C1-3 alkyl and C1-3 fluoroalkyl; with provision; and free bases, acids, pharmaceutically acceptable salts, solvates, solvates of salts, pure stereoisomers, racemic mixts., diastereomeric mixts., scalemic mixts. and tautomeric forms thereof, are claimed. Example compd. II was prepd. by amidation of 2-methyl-1,8-naphthyridine-3- carboxylic acid with 4-chloro-3-trifluoromethylbenzylamine. All the invention compds. were evaluated for their bronchorelaxing efficacy. From the assay, it was detd. that compd. II exhibited a remaining contraction of 8% and 67% at 10 μM and 1 μM, resp.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
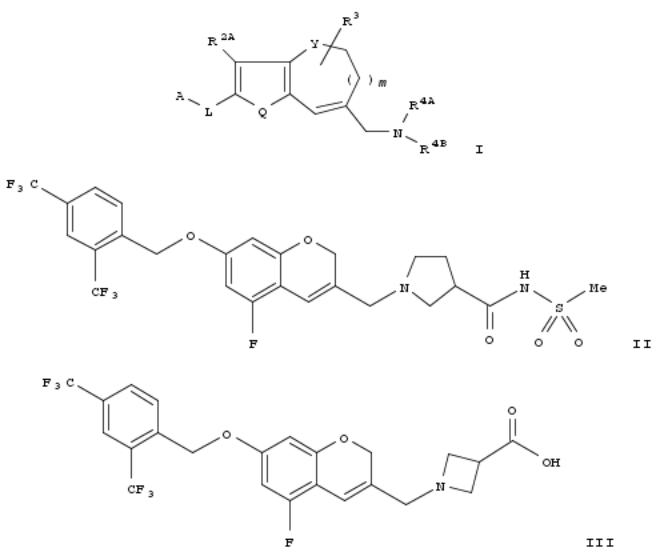
116. Preparation of aminomethyl-2H-chromene compounds and derivative thereof as agonists of S1P1 receptor
By Harada, Hironori; Hattori, Kazuyuki; Fujita, Kazuya; Imada, Sunao; Morokata, Tatsuaki
From PCT Int. Appl. (2010), WO 2010064707 A1 20100610, Language: Japanese, Database: CAPLUS
SciFinder® |
Page 88 |
There are disclosed compds. which have an excellent S1P1 receptor agonistic activity and are particularly useful as active ingredients for prophylactic and/or therapeutic agents for diseases induced by undesirable lymphocyte infiltration or diseases induced by abnormal proliferation or accumulation of cells. There are specifically disclosed 2H-chromene compds. and derivs. thereof represented by formula [I; A = each (un)substituted lower alkyl, cycloalkyl, aryl, or heteroaryl; R1 = halo, cyano, NO2, lower alkyl, lower alkenyl, lower alkynyl, halo-lower alkyl, aryl, heteroaryl, cycloalkyl, HO, lower alkoxy, halo-lower alkoxy, aryloxy, cycloalkyloxy, heteroaryloxy, NH2, monoor di(lower alkyl)amino, halo-lower alkylamino, or cyclic amino, wherein aryl, heteroaryl, cycloalkyl, or cyclic amino is optionally substituted; L = lower alkylene, lower alkenylene, lower alkynylene, -lower alkylene-O, O-lower alkylene, lower alkylene-O-lower alkylene; Q = S, (un)substituted C(R2B):C(R2C); R2A, R2B, R2C = H, halo, lower alkyl, halo-lower alkyl, lower alkoxy, halo-lower alkyl alkoxy; Y = O, S, or CH2, provided that when Y = CH2, Q = S; m = 0,1; R3 = H, halo, lower alkyl, halo-lower alkyl, aryl; R4A = H, lower alkyl; R4B = lower alkyl or cycloalkyl substituted by a group selected from CO2H, tetrazolyl, lower alkyl- S(:O)ONHC(O), CO2-lower alkyl; 5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl; or NR4AR4B = (un)substituted cyclic amino]. These compds. are useful for the prevention or treatment of organ, bone marrow, or tissue transplant rejection, graft-vs.- host diseases, autoimmune disease, or inflammatory diseases, or multiple sclerosis. Thus, a soln. of 98 mg 1-[(7-[[2,4- bis(trifluoromethyl)benzyl]oxy]-5-fluoro-2H-chromen-3-yl)methyl]pyrrolidine-3-carboxylic acid in 2 mL DMF was treated with 46 mg CDI, stirred at 70° for 12 h, treated with 27 mg methanesulfonamide and 43 mg DBU, and stirred for 12 h to give 1-[(7-[[2,4-bis(trifluoromethyl)benzyl]oxy]-5-fluoro-2H-chromen-3-yl)methyl]-N-(methylsulfonyl)pyrrolidine-3- carboxamide (II). A compd. (III) showed ED50 of 0.010 mg/kg p.o. after 4 h for decreasing lymphocyte of peripheral blood in rats.
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
117. Synthesis and immobilization of amylose derivatives bearing a 4-tert-butylbenzoate group at the 2-position and 3,5- dichlorophenylcarbamate/3-(triethoxysilyl)propylcarbamate groups at 3- and 6-positions as chiral packing material for HPLC
SciFinder® |
Page 89 |
By Shen, Jun; Ikai, Tomoyuki; Shen, Xiande; Okamoto, Yoshio
From Chemistry Letters (2010), 39(5), 442-444. Language: English, Database: CAPLUS, DOI:10.1246/cl.2010.442
Two amylose derivs. bearing 4-tert-butylbenzoate at the 2-position and 3,5-dichlorophenylcarbamate/3- (triethoxysilyl)propylcarbamate residues at 3- and 6-positions were successfully synthesized and immobilized onto silica gel, and their chiral recognition abilities were evaluated as chiral packing materials (CPMs) for HPLC. These immobilized CPMs exhibited recognition ability similar to conventional coated CPM, and for some racemates, comparable or better resolns. were obsd. even compared to com. immobilized amyloseor cellulose-based columns.
~6 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
118. Tryptophan hydroxylase inhibitors for treating osteoporosis
By Shi, Zhi-Cai; Zhao, Matthew Mangzhu
From PCT Int. Appl. (2010), WO 2010062829 A1 20100603, Language: English, Database: CAPLUS
The invention discloses the prepn. and use of tryptophan hydroxylase inhibitors, eg. (S)-2-amino-3-(4-(4-amino-6-((R)-1- (naphthalen-2-yl)ethylamino)-1,3,5-triazin-2-yl)phenyl)propanoic acid, (Markush included), for increasing bone mass and treating diseases and disorders such as osteoporosis.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
119. Profiling Sulfonate Ester Stability: Identification of Complementary Protecting Groups for Sulfonates
By Miller, Stephen C.
From Journal of Organic Chemistry (2010), 75(13), 4632-4635. Language: English, Database: CAPLUS,
DOI:10.1021/jo1007338
Sulfonation is prized for its ability to impart water-soly. to hydrophobic mols. such as dyes. This modification is usually performed as a final step, since sulfonated mols. are poorly sol. in most org. solvents, which complicates their synthesis and purifn. This work compares the intrinsic lability of different sulfonate esters, identifying new sulfonate protecting groups and mild, selective cleavage conditions.
~10 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
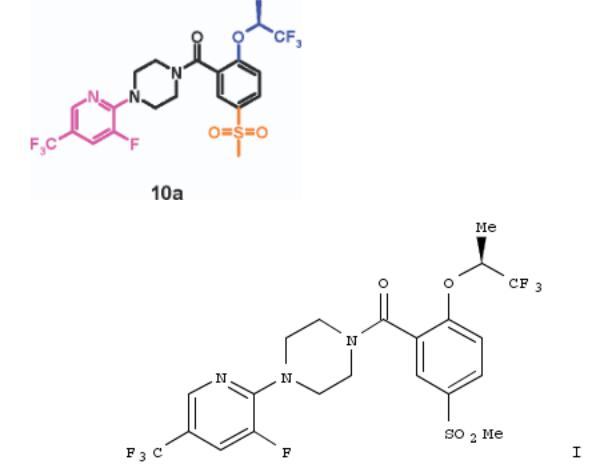
120. Selective GlyT1 Inhibitors: Discovery of [4-(3-Fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl- 2-((S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a Promising Novel Medicine To Treat Schizophrenia
By Pinard, Emmanuel; Alanine, Alexander; Alberati, Daniela; Bender, Markus; Borroni, Edilio; Bourdeaux, Patrick; Brom, Virginie; Burner, Serge; Fischer, Holger; Hainzl, Dominik; et al
From Journal of Medicinal Chemistry (2010), 53(12), 4603-4614. Language: English, Database: CAPLUS, DOI:10.1021/jm100210p
SciFinder® |
Page 90 |
The GlyT1 transporter has emerged as a key novel target for the treatment of schizophrenia. Herein, we report on the optimization of the 2-alkoxy-5-methylsulfonebenzoylpiperazine class of GlyT1 inhibitors to improve hERG channel selectivity and brain penetration. This effort culminated in the discovery of compd. I (RG1678), the first potent and selective GlyT1 inhibitor to have a beneficial effect in schizophrenic patients in a phase II clin. trial.
~43 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
121. Preparation of N-(quinoxalin-2-yl)arylsulfoxamides, N-(quinoxalin-2-yl)sulfamides, and N-(quinoxalin-2- yl)alkanesulfoxamides as kynurenine production inhibitors
By Amishiro, Nobuyoshi; Fukuda, Yuichi; Kinpara, Keisuke; Mie, Motoya; Tagaya, Hisashi; Takahashi, Takeshi From PCT Int. Appl. (2010), WO 2010053182 A1 20100514, Language: Japanese, Database: CAPLUS
There are disclosed kynurenine prodn. inhibitors which contain nitrogen-contg. heterocyclic compd. represented by formula [I; R50, R51 = H, (un)substituted lower alkoxy, halo; or R50C:CR51 together form (un)substituted benzene,
naphthalene, or pyridine ring; G1, G2 = N or CH, provided that G1 = G2 ¹ CH; X = (Y)m1(CR6R7)m2; m1, m2 = 0, 1; Y = S(O)m3, NR8; m3 = an integer of 0-2; R8 = H, (un)substituted lower alkyl; R6, R7 = H, halo, cyano, CO2H, each
(un)substituted lower alkyl, cycloalkyl, aryl, heterocyclyl, lower alkanoyl, lower alkoxycarbonyl, CONH2, or N-contg. heterocyclylcarbonyl; R1 = each lower alkyl, lower alkenyl, lower alkynyl, cycloalkyl, aryl, heterocyclyl, or NH2; R2 = H, (un)substituted lower alkyl; or R1S(O)2NR2 together form (un)substituted heterocyclyl contg. S and N atom; R3 = each (un)substituted lower alkyl, cycloalkyl, aryl, heterocyclyl, lower alkanoyl, lower alkoxycarbonyl, CONH2, or N-contg. heterocyclylcarbonyl] or pharmacol. acceptable salts thereof as the active ingredients. These compds. I have Kynurenine prodn.-inhibitory activity and/or indoleamine 2,3-dioxygenase (IDO)-inhibitory activity and are useful for the prevention and/or treatment of diseases related to the prodn. of kynurenine, in particular cancer. Thus, 2-amino-3-[(2- naphthyl)methoxy]quinoxaline 71.5, 2,3-dichlorobenzenesulfonyl chloride 175, 60% NaH (oil dispersion) 23.7 mg were allowed to react in 1.4 mL THF at room temp. for 28.5 h to give, after purifn. using TLC, 50% 2,3-dichloro-N-[3-[(2- naphthyl)methoxy]quinoxalin-2-yl]benzenesulfonamide (II). II in vitro inhibited the prodn. of kynurenine in human stomach cancer KATO-111 cells treated by IFN-g (which stimulated the prodn. of IDO) with IC50 of 1-10 mM.
