
Reference_01_08_2014_165529
.pdf
SciFinder® |
Page 101 |
The present application describes selective ligands of formula I (wherein X is N or CH; R1 is H, alkyl, alkenyl, or (un)substituted heteroarylalkyl; m and q are 1 or 2; n and p are 0-2; R2 is H, halo, C1-3 haloalkyl, etc.; L1 is a bond, O, S, etc.; R3 is -C2-4alkenylenyl-G1, G2, or -(CRdRe)t-G3; G1, G2, and G3 are aryl, heteroaryl, heterocycle, etc.; t = 1-4) for neuronal nicotinic receptors (NNRs), more specifically for the α4β2 NNR subtype, compns. thereof, and methods of using the same. Synthetic procedures for prepg. I are exemplified. Example compd. II was prepd. in a 9-step reaction sequence that culminated in the reaction of III with 3,5-dimethylaniline followed by deprotection. In an assay that measured binding to α4β2 nAChRs, compds. of the invention had Ki values ranging from 0.01 nM to 1.0 μM (10-11 to 10-6 M).
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
137. New chiral dopant possessing high twisting power
By Pozhidaev, E. P.; Torgova, S. I.; Molkin, V. M.; Minchenko, M. V.; Vashchenko, V. V.; Krivoshey, A. I.; Strigazzi, A. From Molecular Crystals and Liquid Crystals (2009), 509, 1042-1050. Language: English, Database: CAPLUS, DOI:10.1080/15421400903054667
The title compd. was obtained by a reaction of [1,1':4',1''-terphenyl]-4,4''-dicarbonyl dichloride with (R)-(+)-1,1,1-trifluoro- 2-octanol. This compd. [i.e., [1,1':4',1''-terphenyl]-4,4''-dicarboxylic acid 4,4''-bis[(1R)-1-(trifluoromethyl)octyl] ester] is a chiral dopant. This terphenyldicarboxylic acid deriv. has twisting power in a smectic C* mixt. which is much higher than
any other chiral compd. of this series. The ferroelec. liq. crystal mixts. based on an achiral SmC matrix and the new chiral dopant were elaborated and their optical and electrooptical properties were detd.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
138. Fused heterocyclic compounds and compositions for arthropod control
SciFinder® |
Page 102 |
By Iwakoshi, Mitsuhiko; Uemura, Ippei
From PCT Int. Appl. (2009), WO 2009131237 A1 20091029, Language: Japanese, Database: CAPLUS
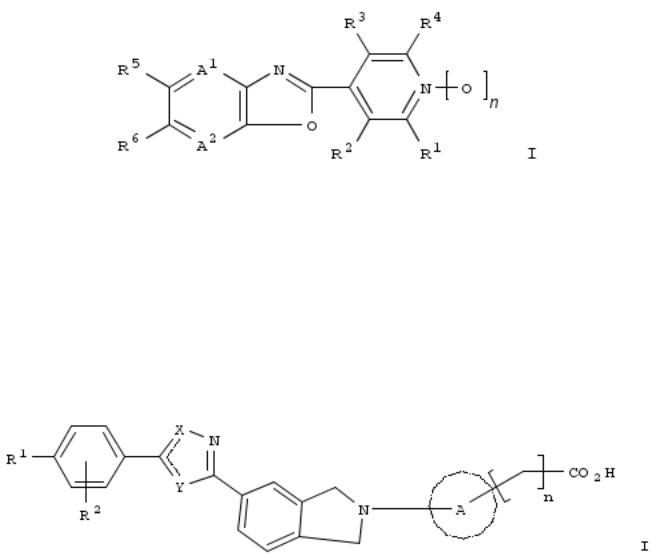
A harmful arthropod control compn. with excellent efficacy comprises, as an active ingredient, a fused heterocyclic compd. (I, wherein A1, A2 independently = N, CR7; R1, R4 independently = H, halo; R2, R3 independently = H, halo, CN, etc.; R5, R6 independently = (un)substituted, linear C1-6 hydrocarbyl or the like (provided that R5 and R6 are not H simultaneously); R7 = (halo)alkyl, etc.; n = 0 or 1). Thus, when a flowable formulation of I (A1, A2 = CH; R1-R4, R6 = H; R5 = iso-Pr; n = 0; prepd.) was dild. to 500 ppm and applied to cucumber seedlings, control of Aphis gossypii was ³90%.
~14 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
139. Isoindoline compounds as S1P1 receptor agonists for treatment of immune disorders
By Harada, Hironori; Hattori, Kazuyuki; Fujita, Kazuya; Morita, Masatada; Imada, Tadashi; Hokata, Tatsuaki From Jpn. Kokai Tokkyo Koho (2009), JP 2009249363 A 20091029, Language: Japanese, Database: CAPLUS
Title compds. have a structure I [X = N, O; Y = N, O, S; if X or Y = O, then the other ¹ O; A = cycloalkane ring, cycloalkene ring, optionally substituted with lower alkyl; R1 = lower (halo)alkyl, (halo)cycloalkyl, (halo)aryloxy, etc.; R2 = H, cyano, halo, lower haloalkyl; n = 0, 1, 2]. I are useful for treatment of GVDH, autoimmune diseases, rheumatoid arthritis, pancreatitis, hepatitis, diabetes, asthma, atopic dermatitis, reperfusion disorders, etc. Thus, cis-4-[5-[5-[3- chloro-4-[2-fluoro-1-(fluoromethyl) ethoxy] phenyl]-1,3,4-thiadiazol-2-yl]-1,3-dihydro-2H-isoindol-2-yl] cyclohexane carboxylic acid hydrochloride showed EC50 of 7.2 when tested in [g-35S]GTP binding assay for human S1P1 (sphingosine-1-phosphate receptor 1).
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
140. Preparation of phenylsulfonylureas as agrochemical herbicides
By Rosinger, Christoph Hugh; Feucht, Dieter; Mueller, Klaus-Helmut; Haeuser-Hahn, Isolde; Dittgen, Jan; Gesing, Ernst Rudolf; Waldraff, Christian
From PCT Int. Appl. (2009), WO 2009127378 A1 20091022, Language: German, Database: CAPLUS
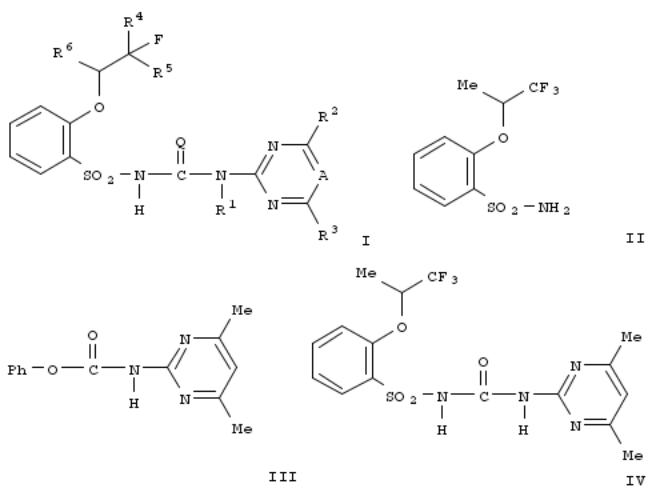
Title compds. I [A = N, CR7; R7 = H, alkyl, halo, etc.; R1 = H, alkyl, alkoxy, etc.; R2 = H, halo, alkyl, etc.; R3 = H, halo, alkyl, etc.; R4 = H, halo, alkyl; R5 = H, halo, alkyl; R6 = alkyl; Q = O, S] were prepd. For example, condensation of sulfonamide II and carbonate III afforded phenylsulfonylurea IV in 96.7% yield. In alopecurus myosuroides protection assays, 6 examples of compds. I exhibited 80-90% inhibition after 3 wk at 80 g/ha.
SciFinder® |
Page 103 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
141. Preparation of novel 7-substituted derivatives of 3-carboxyoxadiazinoquinolones and their use as antibacterial agents
By Ciapetti, Paola; Chery, Mozziconacci Florence; Wermuth, Camille Georges; Leblanc, Francoise; Schneider, Marc; Ropp, Sandrine; Morice, Christophe; Giethlen, Bruno
From Fr. Demande (2009), FR 2928150 A1 20090904, Language: French, Database: CAPLUS
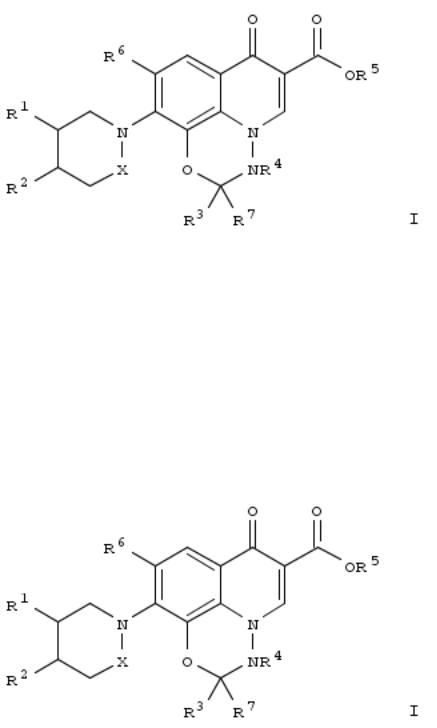
The invention relates to prepn. of tricyclic compds. I [R1 = H, OH, NH2, (CH2)m-NRaRb, m = 0, 1, 2, Ra, Rb = H, linear or branched or cyclic (C1-C6)alkyl, (C3-C6)cycloalkyl-(C1-C6)alkyl, etc.; R2 = H, (CH2)m-NRaRb, Rc, CHReRc, CHReRd, Rc = satd. or unsatd. 5- or 6-membered arom. where 1 to 4 heteroatoms are N, O, and S, Rd = linear or branched (C1-C6)alkyl, (C3-C6)cyclic compd. substituted with 1 to 4 halogens, Re = H, OH , NH2, NH-(C1-C6)alkyl, etc.; R3, R7 = H, (C1-C6)alkyl substituted by 1 to 3 halogens, (C1-c6)alkoxycarbonyl; R4 = Me, substituted by 1 to 3 halogens; R5 = H, (C1-C6)alkyl, (C7- C12)arylakyl; R6 = H, F, NO2, CF3, CN] and their use as antibacterial agents.
SciFinder® |
Page 104 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
142. Preparation of novel 7-substituted derivatives of 3-carboxyoxadiazinoquinolones and their use as antibacterial agents
By Ciapetti, Paola; Chery-Mozziconacci, Florence; Wermuth, Camille G.; Leblanc, Francoise; Schneider, Marc; Ropp, Sandrine; Morice, Christophe; Giethlen, Bruno
From PCT Int. Appl. (2009), WO 2009106967 A1 20090903, Language: French, Database: CAPLUS
The invention relates to prepn. of tricyclic compds. I [R1 = H, OH, NH2, (CH2)m-NRaRb; m = 0, 1, 2; Ra, Rb = H, linear or branched or cyclic (C1-C6)alkyl, (C3-C6)cycloalkyl-(C1-C6)alkyl, etc.; R2 = H, (CH2)m-NRaRb, Rc, CHReRc, CHReRd; Rc = satd. or unsatd. 5- or 6-membered arom. where 1 to 4 heteroatoms are N, O, and S; Rd = linear or branched (C1-C6)alkyl, (C3-C6)cyclic compd. optionally substituted with 1 to 4 halogens; Re = H, OH , NH2, NH-(C1-C6)alkyl, etc.; R3, R7 = H, (C1-C6)alkyl optionally substituted by 1 to 3 halogens, (C1-C6)alkoxycarbonyl; R4 = Me, optionally substituted by 1 to 3 halogens; R5 = H, (C1-C6)alkyl, (C7-C12)arylalkyl; R6 = H, F, NO2, CF3, CN] and their use as antibacterial agents.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
143. Substituted 3-(4-(1,3,5-triazin-2-yl)-phenyl)-2-aminopropanoic acids as novel tryptophan hydroxylase inhibitors
By Jin, Haihong; Cianchetta, Giovanni; Devasagayaraj, Arokiasamy; Gu, Kunjian; Marinelli, Brett; Samala, Lakshman; Scott, Sheldon; Stouch, Terry; Tunoori, Ashok; Wang, Ying; et al
From Bioorganic & Medicinal Chemistry Letters (2009), 19(17), 5229-5232. Language: English, Database: CAPLUS, DOI:10.1016/j.bmcl.2009.07.005
SciFinder® |
Page 105 |
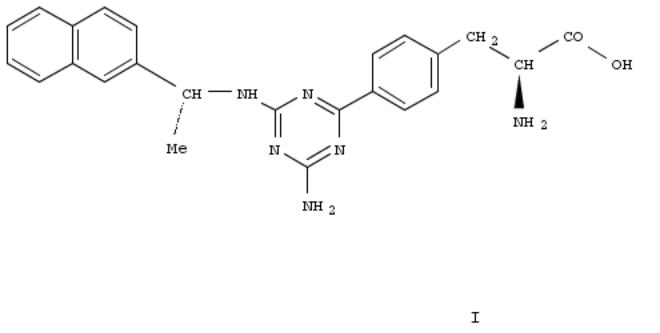
Tryptophan hydroxylase (TPH) is a key enzyme in the synthesis of serotonin. As a neurotransmitter, serotonin plays important physiol. roles both peripherally and centrally. Here we describe the discovery of substituted triazines such as 7b (I) as a novel class of tryptophan hydroxylase inhibitors. This class of TPH inhibitors can selectively reduce serotonin levels in murine intestine after oral administration without affecting levels in the brain. These TPH inhibitors may provide novel treatments for gastrointestinal disorders assocd. with dysregulation of the serotonergic system, such as chemotherapy-induced emesis and irritable bowel syndrome.
~9 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
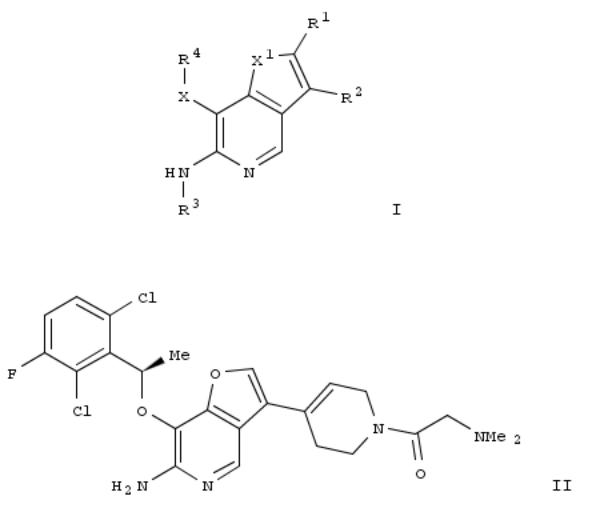
144. Preparation of furo[3,2-c]pyridines and thieno[3,2-c]pyridines as RON and c-Met protein kinase inhibitors for treating neoplasm
By Li, An-Hu; Steinig, Arno G.; Kleinberg, Andrew; Weng, Qinghua; Mulvihill, Mark J.; Wang, Jing; Chen, Xin; Wang, Ti; Dong, Hanqing; Jin, Meizhong
From U.S. Pat. Appl. Publ. (2009), US 20090197864 A1 20090806, Language: English, Database: CAPLUS
The invention is related to the prepn. of title compds. I [X1 = O, S; X = (X2)q; q = 0-1; X2 = S(O)0-2, NH and derivs.; R1, R2 = independently H, halo, CN, NO2, aryl, etc.; or R2 = (un)substituted 1,2,3,6-tetrahydropyridin-4-yl; R3 = H, alkyl; R4 = H, arylcycloalkyl, alkyl, arylalkyl, etc.; or R4 = (CH2)0-7A1; A1 = (un)substituted (hetero)aryl] their pharmaceutically acceptable salts, their pharmaceutical compns. and their use, in treatment of cancers, including conditions in which EMT is involved, including conditions mediated by protein kinase activity such as RON and/or MET. Thus, a multi-step synthesis from 3-Bromo-7-[[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethyl]oxy]-6-nitrofuro[3,2-c]pyridine was given for furopyridine II. II inhibited RON and Met kinases with IC50 ≤ 0.5 μM.
SciFinder® |
Page 106 |
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
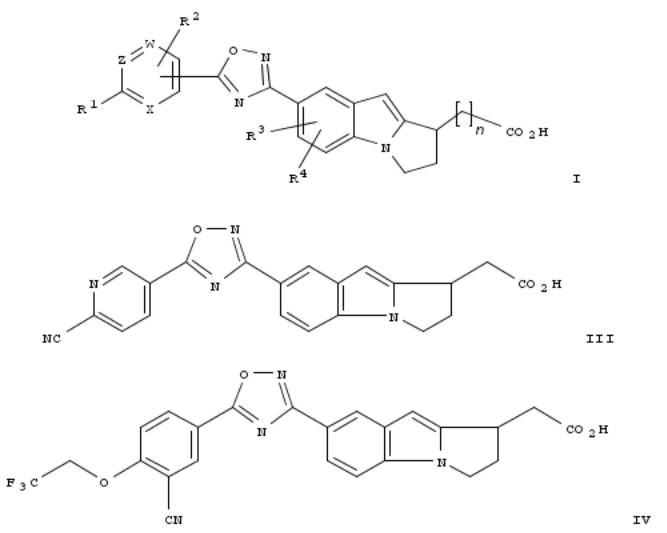
145. Preparation of dihydro-1H-pyrrolo[1,2-a]indol-1-yl carboxylic derivatives which act as S1P1 receptor agonists
By Jones, Robert M.; Buzard, Daniel J.; Kawasaki, Andrew M.; Lopez, Luis A.; Moody, Jeanne V.; Thoresen, Lars; Ullman, Brett
From PCT Int. Appl. (2009), WO 2009094157 A1 20090730, Language: English, Database: CAPLUS
SciFinder® |
Page 107 |
The present invention relates to certain (1,2,4-oxadiazol-3-yl)-2,3-dihydro-1H-pyrrolo[1,2-a]indol-1-yl carboxylic acid derivs. of formula [I; n = 0-1; W = N or CR5; Z = N or CR6; X = N or CR7; provided that W, Z and X are not all N; R1, R2, R5, R6, R7 = H, C1-6 acyl, C1-6 acyloxy, C1-6 alkoxy, C1-6 alkoxycarbonylamino, C1-6 alkyl, C2-6 alkynyl, C1-6 alkylamino, C2-C8 dialkylamino, C1-6 alkylcarboxamide, C1-6 alkylsulfonamide, C1-6 alkylsulfinyl, C1-6 alkylsulfonyl, C1-6 alkylthio, amino, carboxamide, carboxy, cyano, C3-7 cycloalkyl, C3-7 cycloalkyloxy, C3-7 cycloalkylthio, C3-7 cycloalkylsulfinyl, C3-7 cycloalkylsulfonyl, C2-6 dialkylcarboxamide, C1-6 haloalkoxy, C1-6 haloalkyl, C1-6 haloalkylsulfinyl, C1-6 haloalkylsulfonyl, halogen, heteroaryl, heterocyclyl, hydroxy, nitro, or sulfonamide, etc., wherein C1-6 alkoxy, C1-6 alkylthio, C1-6 alkyl, C2-6 alkynyl and heteroaryl are optionally substituted; or two adjacent groups selected from R1, R2, R5, R6 and R7 together with the atoms to which they are both bonded form (un)substituted five or six member heterocyclyl ring optionally substituted with one or two halogen atoms; R3, R4 = H, C1-2 alkyl, F, Cl] and pharmaceutically acceptable salts thereof. These compds. exhibit useful pharmacol. properties, for example, as agonists of the sphingosine-1-phosphate receptors EDG-1 (S1P1 receptor). There are also provided by the present invention, pharmaceutical compns. contg. compds. of the invention, and methods of using the compds. and compns. of the invention in the treatment of S1P1 assocd. disorders, for example, psoriasis, rheumatoid arthritis, Crohn's disease, transplant rejection, multiple sclerosis, systemic lupus erythematosus, ulcerative colitis, type I diabetes, sepsis, myocardial infarction, ischemic stroke, acne, microbial infections or diseases and viral infections or diseases. Thus, tert-Bu 2-[7-[5-(6-cyanopyridin-3-yl)-1,2,4-oxadiazol-3-yl]- 2,3-dihydro-1H-pyrrolo[1,2-a]indol-1-yl]acetate >. thus, a soln. of tert-Bu 2-[7-(N'-hydroxycarbamimidoyl)-2,3-dihydro-1H- pyrrolo[1,2-a]indol-1-yl]acetate (50 mg) in dioxane (914 μL) and 6-cyanonicotinic acid (45.0 mg) was treated with Et3N (212 μL) followed by addn. of 1-propylphosphonic acid cyclic anhydride (49.2 μL), stirred for 30 min, heated to reflux for 18 h, dild. with water, and filtered to give tert-Bu 2-[7-[5-(6-cyanopyridin-3-yl)-1,2,4-oxadiazol-3-yl]-2,3-dihydro-1H- pyrrolo[1,2-a]indol-1-yl]acetate (II) as a yellow solid. A soln. of 43.1 mg II in CH2Cl2 (325 μL) was treated with thioanisole (107 μL) and CF3CO2H (150 μL), stirred for 1 h, evapd. under reduced pressure to give, after purifn. using preparative HPLC, 2-[7-[5-(6-cyanopyridin-3-yl)-1,2,4-oxadiazol-3-yl]-2,3-dihydro-1H-pyrrolo[1,2-a]indol-1-yl]acetic acid (III). 2-[7-[5- [3-Cyano-4-(2,2,2-trifluoroethoxy)phenyl]-1,2,4-oxadiazol-3-yl]-2,3-dihydro-1H-pyrrolo[1,2-a]indol-1-yl]acetic acid (IV), similarly prepd. from tert-Bu 2-[7-(N'-hydroxycarbamimidoyl)-2,3-dihydro-1H-pyrrolo[1,2-a]indol-1-yl]acetate and 3-cyano- 4-(2,2,2-trifluoroethoxy)benzoic acid, in vitro decreased the concn. of endogenous cAMP produced by CHO-K1 cells with ED50 of 6.3 nM.
~3 Citings
SciFinder® |
Page 108 |
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
146. Kinetic resolution of fluorinated propargyl alcohols by lipase-catalyzed enantioselective transesterification
By Ko, Sung-Jin; Lim, Jung Yun; Jeon, Nan Young; Won, Keehoon; Ha, Deok-Chan; Kim, Bum Tae; Lee, Hyuk From Tetrahedron: Asymmetry (2009), 20(10), 1109-1114. Language: English, Database: CAPLUS, DOI:10.1016/j.tetasy.2009.03.041
In order to obtain optically active fluorinated propargyl alcs., a lipase-catalyzed kinetic resoln. has been carried out. The effect of lipase types, org. solvents, reaction temp., and acyl donors was examd. in the lipase-catalyzed transesterification of 1,1,1-trifluoro-4-phenyl-3-butyn-2-ol. Various enantiomerically pure fluorinated propargyl alcs. have been successfully prepd. in good enantiomeric excess (>84%) by Novozym 435-catalyzed transesterification with vinyl butanoate at 60 °C in n-hexane. In some cases, the enantiomeric purities were excellent (>99% ee).
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
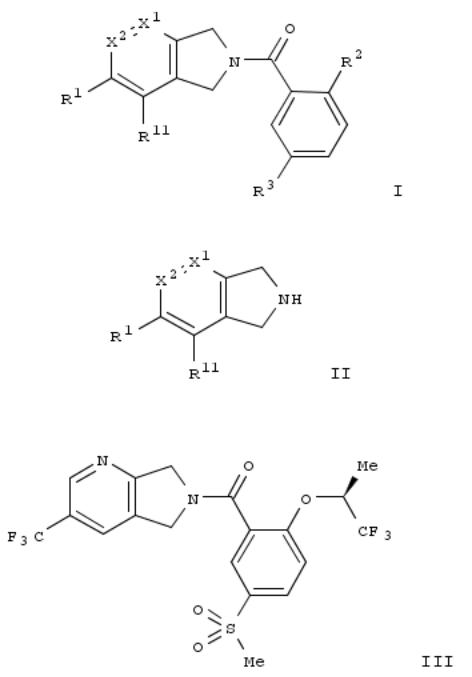
147. Preparation of dihydropyrrole derivatives as intermediates
By Pfleger, Christophe; Spurr, Paul; Trussardi, Rene; Waldmeier, Pius; Wang, Shaoning
From PCT Int. Appl. (2009), WO 2009068463 A2 20090604, Language: English, Database: CAPLUS
The invention is concerned with a scalable process for the prepn. of compds. of formula I comprising a process for the prepn. of the key intermediate, a dihydropyrrole derivs. of formula II and their salts. The process for the prepn. of compds. of formula I via amidation of substituted benzoic acids with compds. of formula II was claimed. Compds. of formulas I and II wherein X1 is N and C; X2 is C; when X1 is C, then X2 is C and N; R1 and R11 are independently H, OH, halo, (un)substituted lower alkyl, (un)substituted lower alkoxy, cycloalkyl, CN, NH2, lower alkylthio, lower alkylsulfonyl, etc.; R2 is halo, (un)substituted lower alkyl, (un)substituted lower alkoxy, (hetero)cycloalkyl, NH2 and derivs., cyclic amine, aryl and 5- to 6-membered heteroaryl; R3 is lower alkylsulfonyl, lower alkylaminosulfonyl, NO2 and CN; are claimed. Example compd. III was prepd. via carboxylation of 2,3-dichloro-5-(trifluoromethyl)pyridine; the resulting Et 5- (trifluoromethyl)pyridine-2,3-dicarboxylate underwent redn. to give 2,3-di(hydroxymethyl)-5-(trifluoromethyl)pyridine, which underwent mesylation to give the corresponding mesylate, which underwent cyclocondensation with diphenylmethylamine to give II, which underwent salt formation to give II·HCl, which underwent acylation with (S)-5- methanesulfonyl-2-(1,1,1-trifluoro-2-propoxy)benzoic acid to give I.
SciFinder® |
Page 109 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
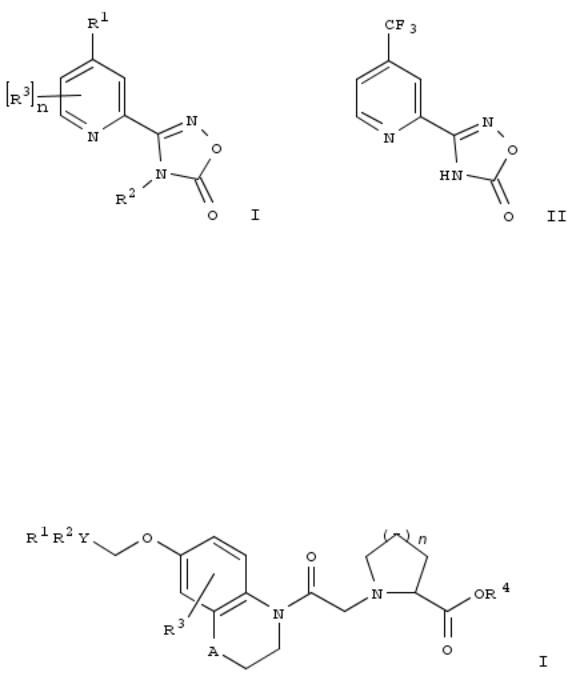
148. Preparation of pyridinyl oxadiazolones for controlling pests
By Mizuno, Hajime
From PCT Int. Appl. (2009), WO 2009066786 A1 20090528, Language: English, Database: CAPLUS
The title compds. I [R1 = (un)substituted haloalkyl, haloalkoxy; R2 = H, cyanomethyl, alkyl, etc.; R3 = alkyl, alkoxy, haloalkoxy; n = 0-3], useful for controlling a pest by applying an effective amt. of the pyridine compd. I to the pest or a place where the pest inhabits, were prepd. and claimed. Thus, reacting 4-trifluoromethylpyridine-2-carboxamide-oxime (prepn. given) with 1,1'-carbonyldiimidazole afforded II.
SciFinder® |
Page 110 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
149. Preparation of azacyclo-alkanecarboxylic acids, their pharmaceutical compositions, their use, and method for therapy
By Yoshino, Toshiharu; Chiba, Atsushi; Suzuki, Takashi; Goto, Taiji; Machinaga, Nobuo
From Jpn. Kokai Tokkyo Koho (2009), JP 2009114141 A 20090528, Language: Japanese, Database: CAPLUS
Title compds. I (A = single bond, O; Y = Ph, thienyl; R1 = C1-6 haloalkyl, C1-6 haloalkoxy; R2 = C1-6 alkoxy, C1-6 haloalkoxy, C3-8 cycloalkyl, C3-8 cycloalkenyl, C3-8 cycloalkylmethyl, C3-8 cycloalkyloxy, C3-8 cycloalkylmethoxy; R3, R4 = H, C1-6 alkyl; n = 1, 2), their pharmacol. acceptable salts, or solvates are prepd. The compds. are useful for prophylactic and/or therapeutic treatment of transplant rejection, autoimmune diseases, and/or allergy. Also claimed are pharmaceutical compns. contg. the compds. and immunosuppressants, immuno-suppressing antibodies, antibiotics, and/or steroids. Thus, treatment of 4-cyclohexyl-3-trifluoromethylbenzyl chloride with Et (R)-1-[2-oxo-2-(5- hydroxyindolin-1-yl)ethyl]piperidine-3-carboxylate and K2CO3 at 60° for 4 h in DMF gave the corresponding ether, which was hydrolyzed to afford (R)-I (A = bond, R1R2Y = 4-cyclohexyl-3-trifluoromethylphenyl, R3 = R4 = H, n = 2), which showed sphingosine-1-phosphate receptor agonist activity with EC50 value of 78.0 nM in mice.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
150. Preparation of bicyclic heterocycle derivatives as GPR119 modulators
By Xia, Yan; Boyle, Craig D.; Greenlee, William J.; Chackalamannil, Samuel; Jayne, Charles Lee; Stamford, Andrew W.; Dai, Xing; Harris, Joel M.; Neustadt, Bernard R.; Neelamkavil, Santhosh Francis; et al
From PCT Int. Appl. (2009), WO 2009055331 A2 20090430, Language: English, Database: CAPLUS
