
Reference_01_08_2014_165529
.pdf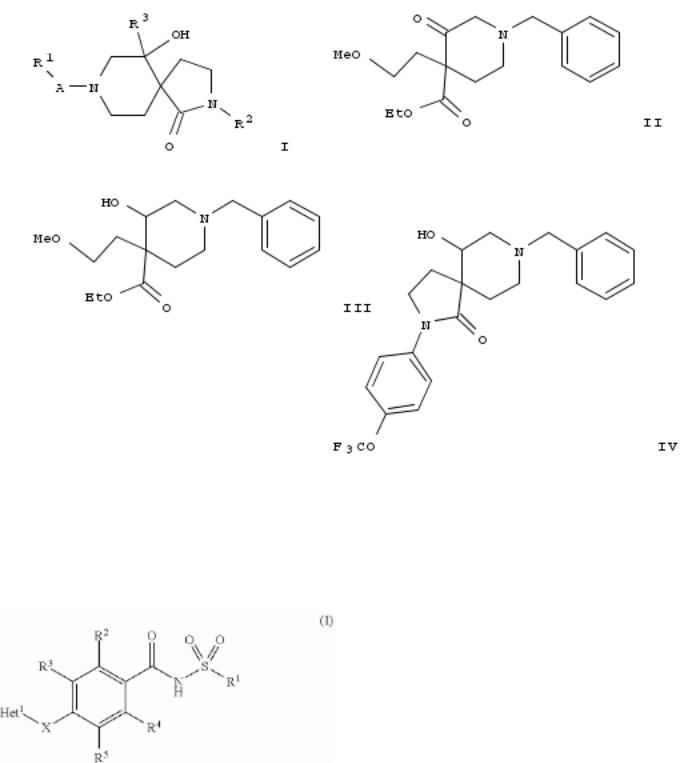
SciFinder® |
Page 51 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
65. Preparation of heteroaryl sulfonamide derivatives as Nav1.7 inhibitors
By Bell, Andrew Simon; Brown, Alan Daniel; De Groot, Marcel John; Lewthwaite, Russell Andrew; Marsh, Ian Roger; Millan, David Simon; Pacheco, Manuel Perez; Rawson, David James; Sciammetta, Nunzio; Storer, Robert Ian; et al From U.S. Pat. Appl. Publ. (2012), US 20120010183 A1 20120112, Language: English, Database: CAPLUS
The invention relates to sulfonamide derivs., to their use in medicine, to compns. contg. them, to processes for their prepn. and to intermediates used in such processes. More particularly the invention relates to a new sulfonamide Nav1.7 inhibitors of formula I [X = O, S, NH, CH2; Het1 = 9-10 membered heteroaryl comprising 1-3 N atoms, or (un)substituted 6-, 9- or 10membered heteroaryl comprising 1-3 N atoms; R1 = (cyclo)alkyl (optionally substituted by 1-8 F atoms); R2-R4 = H, Cl, F, OMe; R5 = H, CN, F, Cl, etc.] or a pharmaceutically acceptable salts thereof, potentially useful in the treatment of a wide range of disorders, particularly pain. Over two-hundred-seventy compds. I were prepd. Thus, reacting 4-[(5-chloro-6- isobutoxypyridin-3-yl)oxy]-3-cyanobenzoic acid (prepn. given) with methanesulfonamide afforded 18% II. Exemplified compds. I were tested for their ability to block the Nav1.7 channel (EIC50 values were provided). Pharmaceutical compns. comprising the compd. I, alone or in combination with other therapeutic agent, were disclosed.
SciFinder® |
Page 52 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
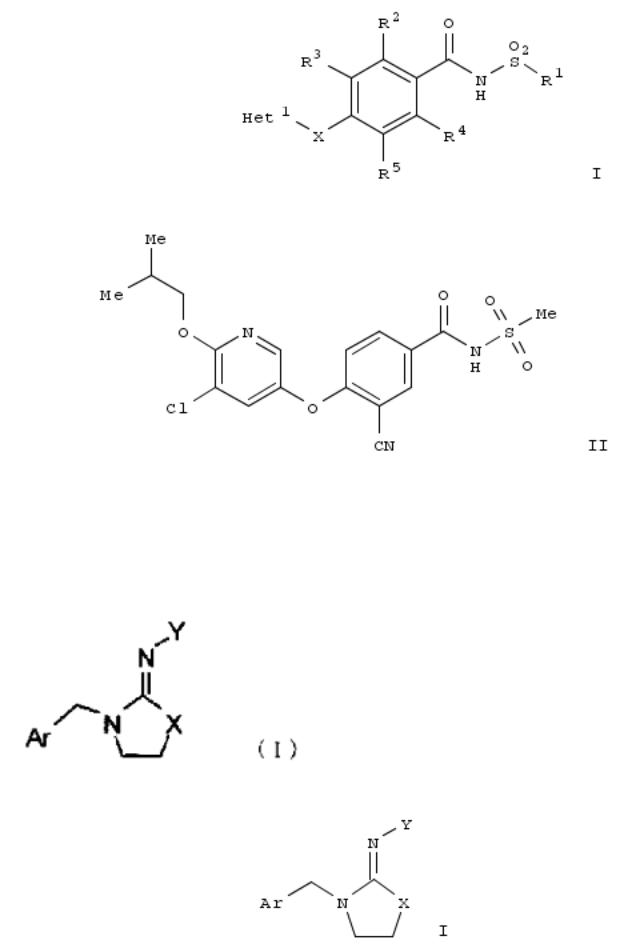
66. Pest control agent comprising imino compound
By Kagabu, Shinzo; Mitomi, Masaaki; Kitsuda, Shigeki; Nomura, Masahiro; Nakamura, Satoshi; Yamamoto, Kazumi From PCT Int. Appl. (2012), WO 2012005216 A1 20120112, Language: Japanese, Database: CAPLUS
Disclosed is a pest control agent which uses at least one kind of imino deriv. represented by chem. formula (I; Ar = (un)substituted heterocyclyl; X = S, CH2 or NR; R = H or alkyl; Y = COR1, CONR3R4, CONHCOR5 and CO2R9; R1-5, R9 = independently H, alkyl, alkenyl, etc.). The imino deriv. can be used for removal of pest, e.g. animal-parasitic ticks and mosquitos, etc. For example, N-[3-[(6-chloro-3- pyridinyl)methyl]-2-thiazolidinylidene]-benzamide was prepd., which killed ³ 60 % of Haemaphysalis longicornis at 200 ppm.
SciFinder® |
Page 53 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
67. Discovery of bacterial NAD+-dependent DNA ligase inhibitors: Improvements in clearance of adenosine series
By Stokes, Suzanne S.; Gowravaram, Madhusudhan; Huynh, Hoan; Lu, Min; Mullen, George B.; Chen, Brendan; Albert, Robert; O'Shea, Thomas J.; Rooney, Michael T.; Hu, Haiqing; et al
From Bioorganic & Medicinal Chemistry Letters (2012), 22(1), 85-89. Language: English, Database: CAPLUS, DOI:10.1016/j.bmcl.2011.11.071
Optimization of clearance of adenosine inhibitors of bacterial NAD+-dependent DNA ligase is discussed. To reduce Cytochrome P 450-mediated metabolic clearance, many strategies were explored; however, most modifications resulted in compds. with reduced antibacterial activity and/or unchanged total clearance. The alkyl side chains of the 2- cycloalkoxyadenosines were fluorinated, and compds. with moderate antibacterial activity and favorable pharmacokinetic properties in rat and dog were identified.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
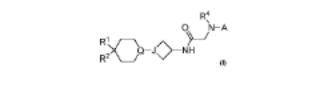
68. Cyclohexyl-azetidinyl derivatives as antagonists of CCR2 and their preparation and use in the treatment of diseases
By Lanter, James C.; Markotan, Thomas P.; Subasinghe, Nalin; Sui, Zhihua
From PCT Int. Appl. (2011), WO 2011159854 A1 20111222, Language: English, Database: CAPLUS
The invention comprises compds. of formula I as CCR2 antagonists and their prepn. The invention also comprises a method of preventing, treating or ameliorating a syndrome, disorder or disease, wherein said syndrome, disorder or disease is type II diabetes, obesity and asthma. The invention also comprises a method of inhibiting CCR2 activity in a mammal by administration of a therapeutically effective amt. of at least one compd. of formula I. Compds. of formula I wherein A is perfluoroalkylisoquinolinyl, trifluoromethylpyridazinyl, trifluoromethylcinnolinyl, etc.; when J is N, then Q is CR5; when J is CH then Q is N; R1 is H, Ph heteroaryl, CF3, etc.; R2 is H and OH; R1R2 may be taken together to form carbonyl; R4 is H and Me; R5 is H and D; and pharmaceutically acceptable salts thereof, are claimed. Example compd. II was prepd. by a multistep procedure (procedure given). All the invention compds. were evaluated for their CCR2 antagonistic activity. From the assay, it was detd. that compd. II exhibited IC50 value of 0.004 μM.
SciFinder® |
Page 54 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
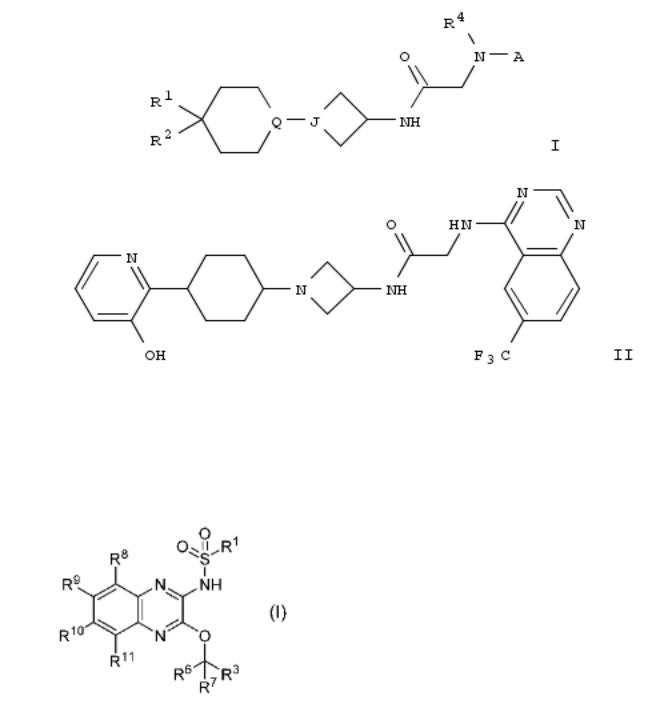
69. Preparation of nitrogen-containing heterocyclic compounds for inhibiting kynurenine production
By Fukuda, Yuichi; Kanai, Toshimi; Nakasato, Yoshisuke; Kimpara, Keisuke
From PCT Int. Appl. (2011), WO 2011142316 A1 20111117, Language: Japanese, Database: CAPLUS
Disclosed are compds. I [R6, R7 = H, (un)substituted alkyl, (un)substituted cycloalkyl, etc.; R8-R11 = H, halo, cyano, etc.; R1 = alkyl (optionally substituted with cycloalkyl) or alkyl (optionally substituted alkoxy); R3 = (un)substituted aryl or (un)substituted heterocyclyl; with provisos; or pharmacol. acceptable salts thereof], useful for the treatment of cancer, immune disease, neurodegeneration, etc. For example, compd. II [R11 = OH] was prepd. via reaction of 2,3- dichloroquinoxaline with propane-1-sulfonamide and treatment with NaH/2-methyl-1-(pyridin-3-yl)propane-1,2-diol. In kynurenine prodn. inhibition assay, compd. II [R11 = methyl] showed ³80% inhibition at 10 mmol/L. Pharmaceutical formulations contg. I are provided.

SciFinder® |
Page 55 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
70. Synthesis of Near-IR Fluorescent Oxazine Dyes with Esterase-Labile Sulfonate Esters
By Pauff, Steven M.; Miller, Stephen C.
From Organic Letters (2011), 13(23), 6196-6199. Language: English, Database: CAPLUS, DOI:10.1021/ol202619f
Near-IR oxazine dyes are reported that contain sulfonate esters which are rapidly cleaved by esterase activity to unmask highly polar anionic sulfonates. Strategies for the synthesis of these dyes included the development of milder dye condensation conditions with improved functional compatibility and the use of an alkyl halide that allows for the introduction of esterase-labile sulfonates without the need for sulfonation of the target mol.
~9 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
71. A simple, rapid procedure for nucleophilic radiosynthesis of aliphatic [18F]trifluoromethyl groups
By Riss, Patrick J.; Aigbirhio, Franklin I.
From Chemical Communications (Cambridge, United Kingdom) (2011), 47(43), 11873-11875. Language: English, Database: CAPLUS, DOI:10.1039/c1cc15342k
A procedure for the radiosynthesis of aliph. [18F]trifluoromethyl groups by reacting 1,1-difluorovinyl precursors with [18F]fluoride ions, resulting in the equiv. of direct nucleophilic addn. of H[18F]F, has been developed. A variety of 18F- labeled model compds. were then obtained and two potential [18F]radiotracers were synthesized by a two step process starting from 1,1-difluorovin-2-yl 4-toluenesulfonate. The method is widely applicable for the synthesis of novel radiotracers in high radiochem. yields and good specific activity.
~11 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
72. Cyclohexyloxypiperidine derivatives as GPR119 receptor modulators and their preparation and use for the treatment of GPR119 receptor-mediated diseases
SciFinder® |
Page 56 |
By Jones, Robert M.; Han, Sangdon; Buzard, Daniel J.; Lehmann, Juerg; Narayanan, Sanju; Yue, Dawei From PCT Int. Appl. (2011), WO 2011127051 A1 20111013, Language: English, Database: CAPLUS
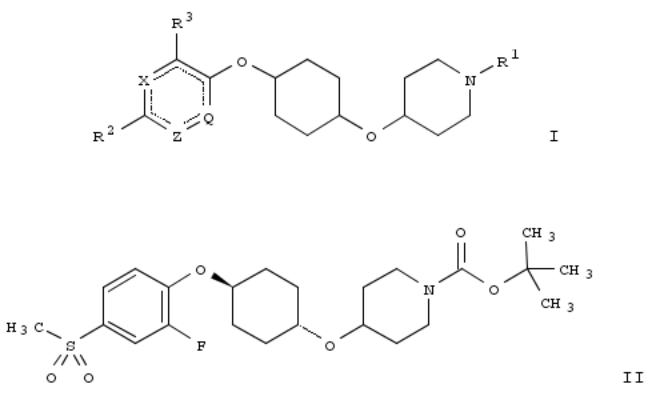
The invention relates to cyclohexyloxypiperidine derivs. of formula I, which are GPR119 receptor modulators and which are useful in treatment of GPR119 receptor-mediated diseases. Compds. of formula I are also useful in combination with one or more addnl. pharmaceutical agents, such as, an inhibitor of DPP-IV, a biguanide, an SGLT2 inhibitor and an α- glucosidase inhibitor. Compds. of formula I wherein Q is N and CR4; Z is N and CR5; X is N, NO, CR6; R1 is H, COC1-6 alkyl, heteroaryl, etc.; R2 is H, (un)substituted C1-6 alkyl, Cn, etc.; R3, R4, R5 and R6 are independently H, C1-6 alkyl, C1-6 alkylsulfonyl and halo; and pharmaceutically acceptable salts, solvates and hydrates thereof, are claimed. Example compd. II was prepd. by etherification of tert-Bu 4-((1r,4r)-4-hydroxycyclohexyloxy)piperidine-1-carboxylate with 1,2- difluoro-4-(methylsulfonyl)benzene. All the invention compds. were evaluated for their GPR119 receptor modulatory activity. From the assay, it was detd. that compd. II exhibited an EC50 value of 8 nM.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
73. Immobilization and chromatographic evaluation of novel regioselectively substituted amylose-based chiral packing materials for HPLC
By Shen, Jun; Li, Pengfei; Liu, Shuangyan; Shen, Xiande; Okamoto, Yoshio
From Chirality (2011), 23(10), 878-886. Language: English, Database: CAPLUS, DOI:10.1002/chir.21000
The regioselectively substituted amylose derivs. bearing a 4-tert-butylbenzoate or 4-chlorobenzoate group at 2-position, and 3,5-dichlorophenylcarbamate and a small amt. of 3-(triethoxysilyl)propylcarbamate groups at 3- and 6-positions were synthesized by a two-step process based on the esterification of 2-position of a glucose unit. The obtained derivs. were effectively immobilized onto macroporous silica gel by intermol. polycondensation of triethoxysilyl groups. Their chiral recognition abilities were evaluated as chiral packing materials (CPMs) for high-performance liq. chromatog. These CPMs showed high chiral recognition as well as the conventional coated-type CPM, and can be used with the eluentscontg. chloroform and THF. With the extended use of these eluents, improvement of chiral recognition and reversed elution orders were realized. For some racemates, the immobilized CPM exhibited ability comparable or better to the com. immobilized amyloseor cellulose-based columns, Chiralpak IA, IB, and IC.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
74. Preparation of thienopyrimidine derivatives for use in treatment and prophylaxis of Mnk1 and Mnk2 kinase-mediated diseases
SciFinder® |
Page 57 |
By Heckel, Armin; Himmelsbach, Frank; Kley, Joerg; Lehmann-Lintz, Thorsten; Redemann, Norbert; Sauer, Achim; Thomas, Leo; Wiedenmayer, Dieter; Black, Phillip; Blackaby, Wesley; et al
From PCT Int. Appl. (2011), WO 2011104340 A1 20110901, Language: English, Database: CAPLUS
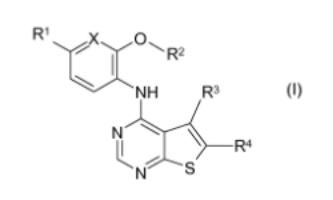
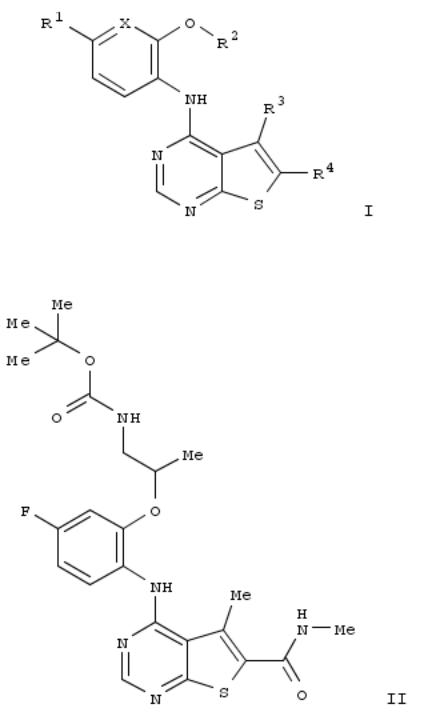
Title compds. I [X = CH or N; R1 = H, halo, CN, alkyl, or CONH2; R2 = (un)substituted alkyl; R3 = alkyl; R4 = CONH2, CONH, alkoxycarbonyl, etc.], and their pharmaceutically acceptable salts, are prepd. and disclosed for use in treatment and prophylaxis of Mnk1 and Mnk2 kinase-mediated diseases. Thus, e.g., II was prepd. by multistep procedure (prepn. given). Select I were evaluated in MNK2 inhibition assays, e.g., II demonstrated an IC50 value of 342 nM.
SciFinder® |
Page 58 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
75. Hydroxyalkylation of 4,5,6,7-tetrahydroindole with polyfluorocarbonyl compounds as a route to 2-substituted indoles
By Sigan, Andrei L.; Gusev, Dmitrii V.; Chkanikov, Nikolai D.; Shmidt, Elena Yu.; Ivanov, Andrei V.; Mihaleva, Al'bina I. From Tetrahedron Letters (2011), 52(39), 5025-5028. Language: English, Database: CAPLUS, DOI:10.1016/j.tetlet.2011.07.071
The regioselective hydroxyalkylation of 4,5,6,7-tetrahydroindole at position 2 using highly electrophilic polyfluorocarbonyl compds. was performed for the first time. Oxidn. of the products thus formed leads to indoles having 2- hydroxypolyfluoroalkyl I(R1 = CF3, R2 = CF3, CO2Et, CH2CO2Et, CF(CF3)2; R1 = CF2CF3, R2 = H) and polyfluoroacyl groups II(R = CF3, CF2CF3, (CF2)5CF2H).

SciFinder® |
Page 59 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
76. Preparation of multicyclic amino acid derivations as potent and selective THP1 inhibitors for treating metastatic bone disease
By Sands, Arthur Thomas
From PCT Int. Appl. (2011), WO 2011100285 A1 20110818, Language: English, Database: CAPLUS
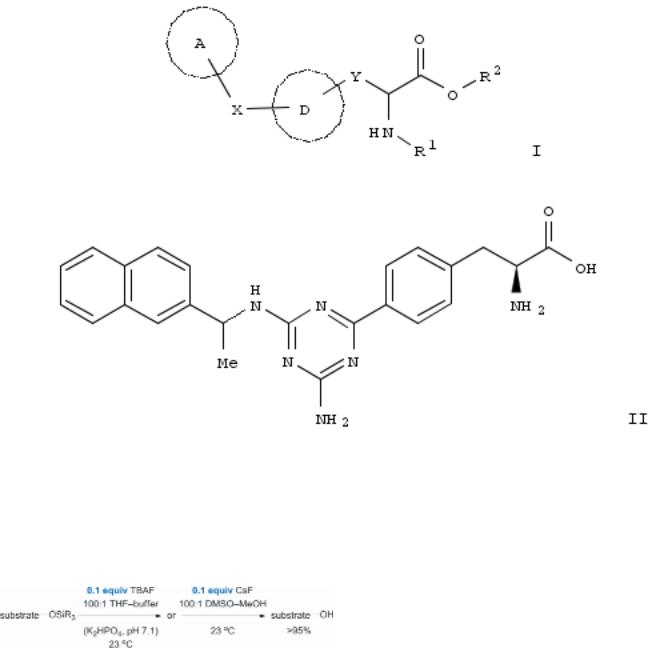
The invention is related to the use of a compd. inhibiting tryptophan hydroxylase (TPH), esp. TPH1 of formula I [A = (un)substituted cycloalkyl, aryl, heterocyclyl; X = a bond, O, S, CO, SO2, NH and derivs., CH2O and derivs., etc.; D = (un)substituted aryl, heterocyclyl; R1, R2 = independently H, (un)substituted alkyl, alkyl/aryl, alkyl/heterocyclyl; Y = (CH2)n; n = 0-3] for treating metastatic bone disease particularly bone metastases of prostate cancer in combination with a second drug (no data). Thus, monoamination of 2-amino-4,6-dichloro-1,3,5-triazine with (R)-(+)-1-(2- naphthyl)ethylamine and Pd-coupling of chloride intermediate (no data) with L-p-boronophenylalanine gave II. Specific compds. of the invention had an IC50 for TPH1 that is about 1000 times less than their IC50 values for TPH2.
SciFinder® |
Page 60 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
77. Use of catalytic fluoride under neutral conditions for cleaving silicon-oxygen bonds
By DiLauro, Anthony M.; Seo, Wanji; Phillips, Scott T.
From Journal of Organic Chemistry (2011), 76(18), 7352-7358. Language: English, Database: CAPLUS, DOI:10.1021/jo200848j
This article describes the development of conditions for cleaving silicon-oxygen bonds using catalytic quantities of fluoride at neutral pH in mixed org.-aq. solns. that contain buffer. A variety of silicon protecting groups can be removed under these conditions, which show tolerance for acidand base-sensitive groups. A modified procedure also is presented using catalytic fluoride in anhyd. DMSO-methanol, which generates primarily volatile silicon byproducts.
~5 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
78. Radiosynthesis of [5-[11C]methanesulfonyl-2-((S)-2,2,2-trifluoro-1-methyl-ethoxy)-phenyl]-[5-(tetrahydro-pyran-4-yl)- 1,3-dihydro-isoindol-2-yl]-methanone ([11C]RO5013853), a novel PET tracer for the(GlyT1)
By Pinard, Emmanuel; Burner, Serge; Cueni, Philipp; Hartung, Thomas; Norcross, Roger D.; Schmid, Philipp; Waldmeier, Pius; Zielinski, Guy; Ravert, Hayden T.; Holt, Daniel P.; et al
From Journal of Labelled Compounds and Radiopharmaceuticals (2011), 54(11), 702-707. Language: English, Database: CAPLUS, DOI:10.1002/jlcr.1911
