
Reference_01_08_2014_165529
.pdf
SciFinder® |
Page 171 |
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
250. A new method for oxidation of various alcohols to the corresponding carbonyl compounds by using N-t- butylbenzenesulfinimidoyl chloride
By Matsuo, Jun-Ichi; Iida, Daisuke; Tatani, Kazuya; Mukaiyama, Teruaki
From Bulletin of the Chemical Society of Japan (2002), 75(2), 223-234. Language: English, Database: CAPLUS, DOI:10.1246/bcsj.75.223
Various primary and secondary alcs. were smoothly oxidized to the corresponding aldehydes and ketones by using a new oxidizing agent, N-t-butylbenzenesulfinimidoyl chloride, together with DBU or ZnO. The present oxidn. proceeded under mild conditions via five-membered intramol. proton-transfer of an alkyl arenesulfinimidate intermediate.
~32 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.

SciFinder® |
Page 172 |
251. Catalytic phosphorylation of polyfluoroalkanols 17. Synthesis and the stereochemistry of tris(α-trifluoromethylbenzyl) phosphates
By Goryunov, E. I.; Petrovskii, P. V.; Shcherbina, T. M.; Zakharov, L. S.
From Russian Chemical Bulletin (Translation of Izvestiya Akademii Nauk, Seriya Khimicheskaya) (2001), 50(6), 10851087. Language: English, Database: CAPLUS
Catalytic phosphorylation of α-trifluoromethylbenzyl alcs., e.g. m-F3CC6H4CH(CF3)OH with POCl3, in the ratio of 3:1 under particular temp. conditions afforded predominantly sym. tris(α-trifluoromethylbenzyl) phosphates, e.g. [m- F3CC6H4CH(CF3)O]3PO. The latter were obtained as mixts. of two diastereomers with a statistical ratio of the components.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
252. Di[(S)-1-(9-anthryl)-2,2,2-trifluoroethyl]sulphite, a case of diastereotopic anthracene groups
By Sanchez, M.; Maestre, I.; Jaime, C.; Virgili, A.
From Tetrahedron: Asymmetry (2001), 12(12), 1737-1740. Language: English, Database: CAPLUS, DOI:10.1016/S0957-4166(01)00286-5
The conformation of di[(S)-1-(9-anthryl)-2,2,2-trifluoroethyl]sulphite 1 was detd. from NMR data and Monte-Carlo conformational search and mol. dynamics calcns. The two diastereotopic ring systems adopt an almost perpendicular relative position.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
253. Preparation of O-substituted 3-fluorovinyloxy(or 3-fluoropropenyloxy) benzaldoximes as agrochemical fungicides
By Kim, Bum Tae; Park, No Kyun; Choi, Gyung Ja; Kim, Jin Cheol; Pak, Chwang Siek
From PCT Int. Appl. (2001), WO 2001012585 A1 20010222, Language: English, Database: CAPLUS
The title compds. [I; X = CH, N; Y = O, NH; R1 = H, alkyl, halo-substituted alkyl; R2 = naphthyl, Ph optionally substituted by alkyl, alkoxy, methylenedioxy, halo; R3 = H, CF3], useful for protecting crops from fungal diseases, were prepd. E.g., a multi-step synthesis of I [X = CH; Y = O; R1 =H; R2 = Ph; R3 = H] was given. Fungicidal activity data for compds. I were presented.
~6 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
254. Lipase-catalyzed optical resolution of trifluoro(aryl)ethanols
By Kato, K.; Gong, Y.-f.; Tanaka, S.; Katayama, M.; Kimoto, H.
From Journal of Molecular Catalysis B: Enzymatic (2001), 11(4-6), 287-294. Language: English, Database: CAPLUS, DOI:10.1016/S1381-1177(00)00046-1
SciFinder® |
Page 173 |
Optical resolns. of racemic 2,2,2-trifluoro-1-(aryl)ethanols - (1-naphthyl), (2-naphthyl), (4-methylnaphthyl), (phenyl), (1- pyrenyl) - were achieved by lipase-catalyzed enantioselective acetylations with vinyl acetate as an acetyl donor in octane, and (S)-acetates and (R)-alcs. were obtained. Among the lipases tested, lipase from Pseudomonas aeruginosa (lipase LIP, Toyobo) showed good enantioselectivity for above ethanols. However, no acetylation occurred with sterically hindered alcs. - (9-phenanthryl), (9-anthryl), (2-methylnaphthyl), (2, 4, 6-trimethylphenyl) - by various lipases. The resolns. of the three alcs. were carried out by the enantioselective alcoholysis or hydrolysis of their chloroacetates by lipase LIP.
~10 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
255. New stable reagents for nucleophilic trifluoromethylation. Part 2: Trifluoromethylation with silylated hemiaminals of trifluoroacetaldehyde
By Billard, T.; Langlois, B. R.; Blond, G.
From Tetrahedron Letters (2000), 41(45), 8777-8780. Language: English, Database: CAPLUS, DOI:10.1016/S0040- 4039(00)01552-5
New reagents for the nucleophilic trifluoromethylation were easily synthesized from fluoral hemiketal. They provided silylated trifluoromethylcarbinol from nonenolizable carbonyl compds.
~40 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
256. Process for separating chiral compounds using a unimodal large pore silica as the support
By McCulloch, Beth; Brandvold, Timothy A.; Nickl, Peter K.; Holmgren, Jennifer S.; Alcaraz, Joseph J. From U.S. (2000), US 6106724 A 20000822, Language: English, Database: CAPLUS
A process for sepg. one chiral compd. from a mixt. of chiral compds. is disclosed. The sepn. process involves using a chiral stationary phase (CSP) which comprises a chiral org. material deposited on an amorphous silica support having a unimodal distribution of pore sizes in the range of 300 Å to 25,000 Å and a surface area of 30 m2 /g.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
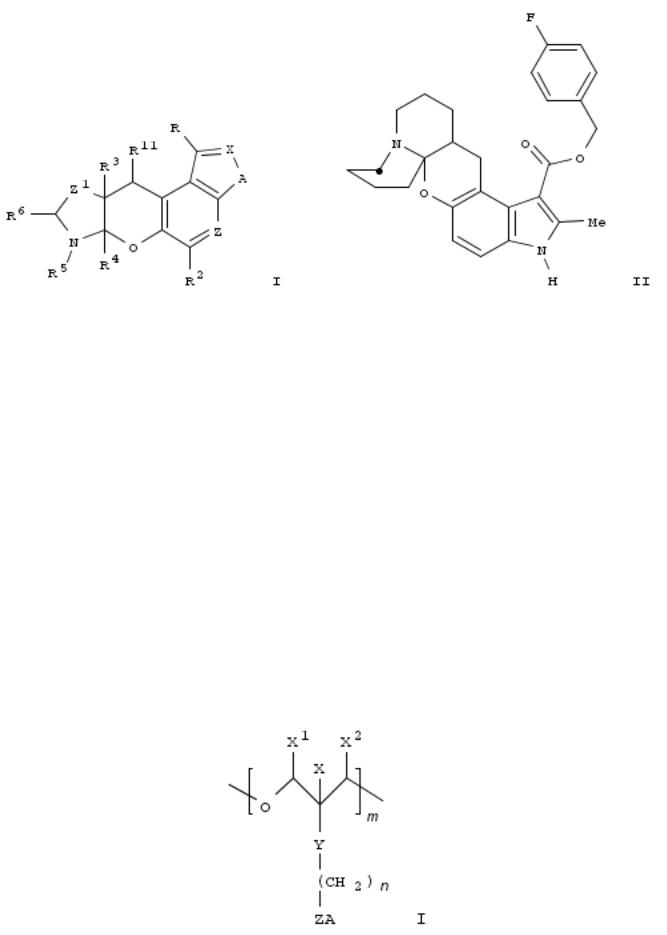
257. Preparation of pyrrolobenzopyranoquinolizinecarboxylates and analogs as CCR-5 chemokine receptor antagonists
By Harriman, Geraldine C.; Kolz, Christine Nylund; Luly, Jay R.; Roth, Bruce David; Song, Yuntao; Trivedi, Bharat Kalidas
From PCT Int. Appl. (2000), WO 2000042045 A2 20000720, Language: English, Database: CAPLUS
Title compds. [I; A = O, S, NR1; R = H, alkyl, aryl(alkyl), CO2H, alkoxycarbonyl, etc.; R1,R3, R6, R11 = H or alkyl; R2 = H, halo, alkyl, alkoxy, etc.; R4 = H, alkyl, aryl(alkyl); R5 = alkyl, aryl(alkyl), acyl; R4R5 = atoms to complete a ring; X = N or CR9; R9 = H, halo, alkyl, alkoxy, etc.; Z = N or (un)substituted CH; Z1 = (CH2)1-3] were prepd. Thus, 4-fluorobenzyl 4- dimethylamino-5-hydroxy-2-methyl-1H-indole-3-carboxylate was was cyclocondensed with 1,2,3,4,6,7,8,9- octahydroquinolizinium perchlorate (prepn each given) to give title compd. II. Data for biol. activity of I were given.
SciFinder® |
Page 174 |
~7 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
258. New catalytic oxidation of trifluoromethyl carbinols by a ruthenium(II) complex
By Kesavan, Venkitasamy; Bonnet-Delpon, Daniele; Begue, Jean-Pierre; Srikanth, Abirami; Chandrasekaran, Srinivasan
From Tetrahedron Letters (2000), 41(18), 3327-3330. Language: English, Database: CAPLUS, DOI:10.1016/S0040- 4039(00)00378-6
The first catalytic oxidn. of trifluoromethyl carbinols was accomplished by a novel Ru(II) complex using NaIO4 as an oxidant. Trifluoromethyl ketones were obtained under mild conditions and in excellent yields.
~22 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
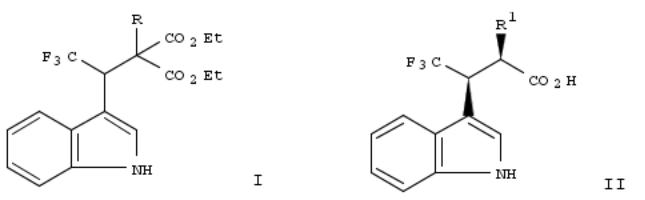
259. Polymeric liquid crystals for display devices
By Toyne, Kenneth Johnson; Cowling, Stephen James; Goodby, John William
From PCT Int. Appl. (1999), WO 9943763 A1 19990902, Language: English, Database: CAPLUS
Polymeric liq. crystals for display devices, sensors, and nonlinear optical instruments are represented by the general formula I wherein X, X1, and X2 are independently selected from straightor branched-chain C1-16 alkyl groups, halogen atoms, and H; A is any suitable mesogenic group; Z is a single covalent bond, oxygen, CO2, or OCO; n = 1-20; Y = oxygen, CO2, OCO, CH2, CHOH, or CH2O; m = 3-10,000 and prepd. by polymn. of oxitane monomers. In this way the mesogenic groups are spaced on every fourth carbon atom in the polymer chain, allowing greater mobility for the mesogenic groups.
SciFinder® |
Page 175 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
260. Lipase-catalyzed optical resolution of trifluoromethylated alcohols and acids
By Kato, Katsuya; Fujii, Shozo; Katayama, Masato; Kimoto, Hiroshi
From Recent Research Developments in Agricultural & Biological Chemistry (1997), 1, 331-343. Language: English, Database: CAPLUS
A highly enantioselective method is developed to resolve racemic trifluoromethylated alcs. and acids by various lipases. The enantioselectivity and reactivity were strongly influenced by the structure of the substrates. Particularly, in the resoln. of 2,2,2-trifluoro-1-(aryl)ethanols, lipase selectivity greatly changed according to the structures of aryl groups. In general, lipase reactivity for fluorinated compds. is lower than that for non-fluorinated counterpart. In this paper the procedure to increase lipase reactivity for fluorinated compds. is described, too.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
261. Highly diastereoselective synthesis of β-trifluoromethyl-N-acetyltryptophan
By Gong, Yuefa; Kato, Katsuya; Kimoto, Hiroshi
From Tetrahedron Letters (1999), 40(31), 5743-5744. Language: English, Database: CAPLUS, DOI:10.1016/S0040- 4039(99)01098-9
Reaction of 1-(indol-3-yl)-2,2,2-trifluoroethanol with RCH(CO2Et)2·Na (R = NHAc, OAc, Me) gave indolyltrifluoroethylcontg. malonate I. I was readily hydrolyzed in an aq. NaOH soln. to form the corresponding disodium salts. Subsequent acidification of the salt resulted in the diastereoselective decarboxylation forming syn-product II (R1 = NHAc, OH) in >89% yields with a 97:3 diastereomeric ratio (for R1 = Me, diastereoselectivity was lower).
~14 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
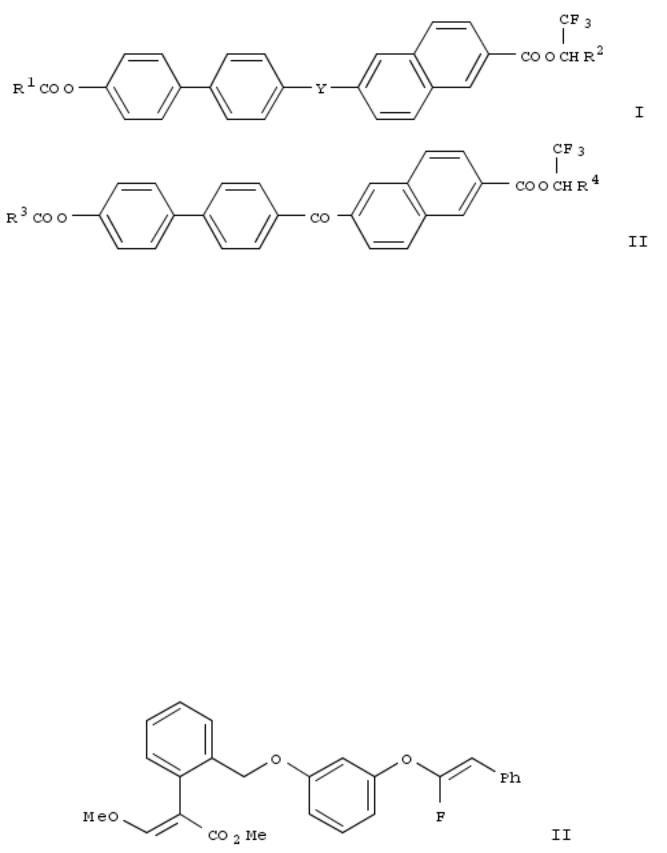
262. Liquid crystal compounds
By Aihara, Yoshihiko; Isozaki, Tadaaki; Yamakawa, Noriko; Kawamura, Ichiro
From U.S. (1999), US 5891359 A 19990406, Language: English, Database: CAPLUS
The novel liq. crystal compds. of the present invention are represented by the formulas I and II (R1 = alkyl having 5-18 carbon atoms; R2 = alkyl having 6-16 carbon atoms; R3 = alkyl having 8-18 carbon atoms; R4 = alkyl having 6-14; Y = CO2 or OCO; * = optically active center), have tristable mol. orientation states, and can be used for display devices and electrooptical devices.
SciFinder® |
Page 176 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
263. Efficient, Ecologically Benign, Aerobic Oxidation of Alcohols
By Marko, Istvan. E.; Giles, Paul R.; Tsukazaki, Masao; Chelle-Regnaut, Isabelle; Gautier, Arnaud; Brown, Stephen M.; Urch, Christopher J.
From Journal of Organic Chemistry (1999), 64(7), 2433-2439. Language: English, Database: CAPLUS, DOI:10.1021/JO982239S
The oxidn. of alcs. into aldehydes and ketones can be efficiently performed using catalytic amts. of CuCl·Phen and mol. oxygen or air. This novel, ecol. friendly procedure releases water as the only byproduct. The oxidn. was performed in the presence of azodicarboxylates or hydrazinodicarboxylates.
~139 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
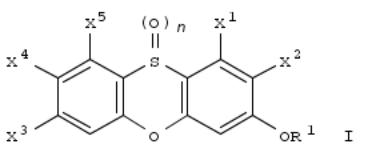
264. Preparation of fluorovinyloxyphenyl moiety-containing methoxyacrylates and iminoacetates as agrochemical fungicides
By Pak, Chwang Siek; Kim, Bum Tae; Park, No Kyun; Choi, Gyung Ja; Kim, Heung Tae
From PCT Int. Appl. (1999), WO 9907665 A1 19990218, Language: English, Database: CAPLUS
RZ1CH2OZ2OCF:CR1R2 [I; R = C(COZMe):XYMe; R1 = H or CF3; R2 = H, alkyl, thienyl, Ph, etc.; X = CH or N; Y = O or S; Z = O or NH; Z1 = 1,2-phenylene; Z2 = 1,3-phenylene] were prepd. Thus, 2-MeC6H4CH2CO2Me was α-formylated and the product O-methylated to give, after isomer sepn. and bromination, (E)-2-(BrCH2)C6H4C(CO2Me):CHOMe which was etherified by 3-(OH)C6H4OH and the product etherified by F2C:CHPh to give title compd. II. Data for biol. activity of I were given.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 177 |
265. Preparation of phenoxathiin dioxides and their monoamine oxidase-A inhibiting ability
By White, Helen Lyng; Boros, Eric; Heyer, Dennis
From PCT Int. Appl. (1998), WO 9812190 A2 19980326, Language: English, Database: CAPLUS
I [n = 0, 1, 2; R1 = branched or straight chain C1-5 alkyl or C3-6 cycloalkyl optionally substituted with hydroxyl or one or more halogens, esp. fluorine; X1, X2, X3, X4, X5 are either all hydrogens or one or two of X1, X2, X3, X4, and X5 are halo and the remainder are hydrogens] were prepd. E.g., reaction of 2-HOC6H4SH and 2,5-F2C6H3NO2 in KOCMe3/DMF gave 3-fluorophenoxathiin, which was oxidized to 3-fluorophenoxathiin 10,10-dioxide. These compds. inhibit monoamine oxidase-A and are useful in the treatment of disorders such as depression.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
266. Enantioselectivity of Pseudomonas cepacia and Candida rugosa lipases for the resolution of secondary alcohols: the effect of Candida rugosa isoenzymes
By Lundell, Katri; Raijola, Timo; Kanerva, Liisa T.
From Enzyme and Microbial Technology (1998), 22(2), 86-93. Language: English, Database: CAPLUS, DOI:10.1016/S0141-0229(97)00104-X
In this work, the lipase (Pseudomonas cepacia) (PS)- and Candida rugosa lipase (CRL)-catalyzed asym. deacylations of the esters of structurally different secondary alcs. were studied. Enantioselectivity in the case of lipase PS was always easily predicted according to the sizes of the substituents at the alc. stereocenter whereas the favored enantiomer in the case of CRL strongly depended on the structure of the racemic alc. moiety in an ester. In the next step, com. CRL was fractionated into its isoenzymes (A and B). The two isoenzymes A and B always gave the same enantiopreferences. This observation clearly showed that the heterogeneity of the com. CRL was not a reason for the reversed enantioselectivity of certain substrates such as cyanohydrin, α-hydroxycarboxylic acid, and 2,2,2-trihaloethanol derivs. when compared to the selectivity obsd. when lipase PS was used as a catalyst.
~40 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
267. Reactions of Alcohols with Cesium Fluoroxysulfate
By Stavber, Stojan; Kosir, Iztok; Zupan, Marko
From Journal of Organic Chemistry (1997), 62(15), 4916-4920. Language: English, Database: CAPLUS, DOI:10.1021/JO962424A
The reactions of alcs. with cesium fluoroxysulfate (CsSO4F) in MeCN suspension were studied, and the role of the structure of the alc. and the reaction conditions on the course of reaction was detd. Secondary benzyl alcs. bearing a nonactivating arom. ring were selectively oxidized to the corresponding ketones, while the CsSO4F-mediated reaction of phenyl-1-naphthylmethanol resulted in the formation of 1-fluoronaphthalene and benzaldehyde. Cyclic and noncyclic secondary alcs. were readily converted to ketones, as well as 1-hydroxybenzocyclanes to benzocyclanones-1, without any further fluorination or oxidn. under the reaction conditions. On the other hand, reactions of primary alcs. with CsSO4F resulted in the formation of acid fluorides derived from further fluorination of aldehydes. Another type of transformation was obsd. in the case of alcs. bearing a benzyl functional group attached geminal to a hydroxy group, where decarbonylation of reactive intermediates resulting in the formation of benzyl fluoride derivs. became the main process. 2-Phenylethanol was so converted to benzyl fluoride and phenylacetyl fluoride in a 3:1 relative ratio, while 2- phenyl-1-propanol was selectively transformed to 1-phenyl-1-fluoroethane. The presence of the radical inhibitor nitrobenzene in the reaction mixt. considerably inhibited conversion of the starting material. The same effect was obsd. by lowering the solvent polarity. Hammett correlation anal. of the effect of substituents on the reaction rates of oxidn. of a set of substituted 1-phenyl-1-ethanols to acetophenones gave the reaction const. ρ+ = -0.32, while anal. of analogous data for the transformations of benzyl alcs. to benzoyl fluorides gave the value of -0.54. A mechanism including radical intermediates was proposed for the transformation of alcs. by CsSO4F.
SciFinder® |
Page 178 |
~11 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
268. Preparation of aldehydes or ketones from alcohols
By Urch, Christopher John; Brown, Stephen Martin; Marko, Istvan Ettienne; Giles, Paul Richard; Tuskazaki, Masao From PCT Int. Appl. (1997), WO 9703033 A1 19970130, Language: English, Database: CAPLUS
A process for prepg. and aldehyde or ketone comprising reacting a primary or secondary alc. with oxygen in the presence of a base, a catalytic amt. of a copper salt and a suitable ligand under anhyd. conditions. Thus, stirring 4- chlorobenzyl alc. with CuCl, KOBu-t, mol. sieves, 1,10-phenanthroline and 1,2-dicarbethoxyhydrazine in PhMe and then bubbling O2 at 80° 45 min gave 100% 4-chlorobenzaldehyde.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
269. Copper-catalyzed oxidation of alcohols to aldehydes and ketones: an efficient, aerobic alternative
By Marko, Istvan E.; Giles, Paul R.; Tsukazaki, Masao; Brown, Stephen M.; Urch, Chistopher J.
From Science (Washington, D. C.) (1996), 274(5295), 2044-2046. Language: English, Database: CAPLUS, DOI:10.1126/science.274.5295.2044
An efficient, copper-based catalyst has been discovered that oxidizes a wide range of alcs. into aldehydes and ketones under mild conditions. This catalytic system utilizes oxygen or air as the ultimate, stoichiometric oxidant, producing water as the only byproduct.
~389 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
270. Highly enantioselective synthesis of long chain alkyl trifluoromethyl carbinols and β-(alkylthio) trifluoromethyl carbinols through lipases
By Petschen, Ines; Malo, Edi A.; Bosch, M. P.; Guerrero, Angel
From Tetrahedron: Asymmetry (1996), 7(7), 2135-2143. Language: English, Database: CAPLUS, DOI:10.1016/0957- 4166(96)00256-X
Among a variety of lipases tested, Candida antarctica lipase has been found to promote the enantioselective acylation of long chain alkyl trifluoromethyl carbinols and β-(alkylthio) trifluoromethyl carbinols, producing both R and S enantiomeric alcs. in good to excellent chem. yield and enantioselectivity. In all cases the lipase preferentially acylates the S enantiomer, irresp. the presence or not of a sulfur atom in β-position to the hydroxyl group. When the reaction was carried out on non-fluorinated substrates, the process occurred much faster and with higher e.e. of the less reacting enantiomer than when conducted on the fluorinated substrates.
~23 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
271. Optical resolution of 2,2,2-trifluoro-1-(9-phenanthryl)ethanol via enzymic alcoholysis of its activated ester
By Kato, Katsuya; Katayama, Masato; Fujii, Shozo; Fukaya, Haruhiko; Kimoto, Hiroshi
From Journal of Fermentation and Bioengineering (1996), 81(3), 206-11. Language: English, Database: CAPLUS, DOI:10.1016/0922-338X(96)82209-4
SciFinder® |
Page 179 |
Practical lipase LIP (Pseudomonas aeruginosa)-catalyzed optical resoln. of 2,2,2-trifluoro-1-(9-phenanthryl)ethanol (TFPE), which is a sterically hindered secondary alc., was performed. The method was used effectively for the first enzymic prepn. of both enantiomers of TFPE via lipase-catalyzed enantioselective alcoholysis of its chloroacetate ester. A simple procedure was developed for sepg. the S-alc. product from the unreactive R-chloroacetate ester and for hydrolyzing the latter to obtain the R-alc. By using a similar procedure, the optical resoln. of 2,2,2-trifluoro-1-(1- naphthyl)ethanol was achieved as well.
~10 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
272. Efficient Synthesis of Optically Pure 1,1,1-Trifluoro-2-alkanols through Lipase-Catalyzed Acylation in Organic Media
By Hamada, Hiroki; Shiromoto, Mizuho; Funahashi, Makoto; Itoh, Toshiyuki; Nakamura, Kaoru From Journal of Organic Chemistry (1996), 61(7), 2332-6. Language: English, Database: CAPLUS, DOI:10.1021/JO951976A
Lipase-catalyzed esterification was successfully utilized for the optical resoln. of 1,1,1-trifluoro-2-alkanols when racemic alkanols were treated with lipase from Candida antarctica in hexane in the presence of mol. sieves (4 Å) to provide the corresponding (S)-acetates in an optically pure state. 1,1,1-Trifluoro-2-alkanols are known as important component of liq. crystal compds. which display remarkable ferroelec. liq. crystal (FLC) characteristics. Five types of optically pure alkanols, i.e., 1,1,1-trifluoro-2-octanol, 1,1,1-trifluoro-2-nonanol, 1,1,1-trifluoro-2-decanol, 1,1,1-trifluoro-2-undecanol, and 1,1,1-trifluoro-8-(benzyloxy)-2-octanol were thus obtained by the lipase-catalyzed acylation.
~40 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
273. Iminoaziridines. Part 6. Ring opening reactions of iminoaziridines and aminoazirinium ions. Is electrocyclic ring opening of 2H-azirinium ions a myth?
By Quast, Helmut; Aldenkortt, Sven; Freudenreich, Bernd; Schaefer, Peter; Peters, Eva-Maria; Peters, Karl; von Schnering, Hans Georg; Wuerthwein, Ernst-Ulrich
From Liebigs Annalen (1996), (1), 87-98. Language: English, Database: CAPLUS
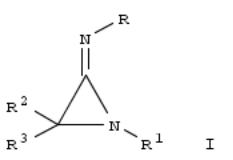
Treatment of the aziridine I [R = R1 = CH2CMe3, R2 = R3 = Me] with HBr yielded similar amts. of the α-bromo amidine BrMe2CC(NHCH2CMe3):NCH2CMe3.HBr and an α,β-unsatd. amidinium bromide. Ring opening reactions of iminoaziridines I [R = Me; R1 = Me, CMe3; R2 = H; R3 = CMe3; II] by nucleophiles were sluggish affording the α-amino imidates Me3CC(NHMe)C(NMe)OR4 [R4 = Me; CH(CF3)R5; R5 = 9-anthryl; 4-O2NC6H4 (III)]. III isomerized via a Meisenheimer-type intermediate to furnish the α-amino amide Me3C[NMe(4-C6H4NO2)]CONHMe. Me triflate converts
(R)-II into the 2-tert-butyl-3-amino-2H-azirinium triflates, which were hydrolyzed without racemization to yield the α-amino amides. In a similar sequence, (R)-II were converted into (R)-α-amino amides by the action of hydrogen halides or 4- MeC6H4SO3H followed by hydrolysis. Some racemization, found in the other cases, was supposed to involve an (R)- imidoyl deriv. In a side reaction, II decompd. into MeNC and an imines Me3CCH:NR1 at low temps. The results are not consistent with the hypothesis of an electrocyclic ring opening of 3-amino-2H-azirinium ions to afford 2-amino-1-azaallyl cations. The heat of protonation of some compds. and structures and energies of the resulting cations were calcd. by ab initio methods. Complete geometry optimizations were performed. Energies were calcd. According to the geometric parameters, the amidine delocalization of the free bases is abandoned in the cations. The calcd. difference in basicity between the two nitrogen atoms of iminoaziridines appears to exclude the ring-protonated cations as intermediates also for reactions in soln.
~6 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 180 |
274. Preparation of Bis(pyrrol-2-yl)halocarbylmethanes
By Wijesekera, Tilak
From Eur. Pat. Appl. (1995), EP 655438 A2 19950531, Language: English, Database: CAPLUS
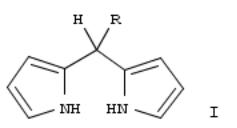
The title compds. (I; R = halocarbyl), useful as intermediates in the prepn. of di-, tri-, tetra-, and polypyrrolic compds. (no data), are prepd. by the direct reaction of pyrrole with a halocarbyl aldehyde RCHO, or by the reaction of pyrrole with a 2- (1-hydroxyl-1-hydro-1-halocarbyl)pyrrole. Thus, heptafluorobutyraldehyde hydrate was reacted with pyrrole in THF the presence of acid catalysts, producing I (R = F3CCF2CF2) in >50% isolated yield.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
275. Enzymic resolution of 1-ferrocenylethanol and 1-ferrocenyl-2,2,2-trifluoroethanol using lipases
By Izumi, Taeko; Aratani, Satoshi
From Journal of Chemical Technology & Biotechnology (1995), 63(1), 25-32. Language: English, Database: CAPLUS, DOI:10.1002/jctb.280630104
1-Ferrocenylethanol and 1-ferrocenyl-2,2,2-trifluoroethanol were resolved with transesterification by lipases to the optically active (R)-esters and (S)-alcs. Enzymic hydrolysis of the racemic esters of 1-ferrocenylethanol using lipases yielded (R)-alc. and (S)-esters. Enantioselective hydrolysis of racemic acetate or butanoate of 1-ferrocenyl-2,2,2- trifluoroethanol yielded the (S)-alc. and (R)-esters. Enzymic hydrolysis of (±)-acetate of 1-ferrocenyl-2,2,2- trifluoroethanol yielded (R)-alc. and (S)-acetate, but the equiv. (±)-butanoate was not hydrolyzed.
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
276. Synthesis and stereochemistry of O-(1-trifluoromethylalkyl) methylchlorophosphonates
By Kabachnik, M. I.; Zakharov, L. S.; Kudryavtsev, I. Yu.; Petrovskii, P. V.; Shcherbina, T. M.; Laretina, A. P.
From Izvestiya Akademii Nauk, Seriya Khimicheskaya (1994), (10), 1840-1. Language: Russian, Database: CAPLUS
1-(Trifluoromethyl)alkanols react with methylphosphonic dichloride in the presence of LiCl or MgCl2 catalyst to give CF3CHROP(O)MeCl (R = Bu, Me3C, n-hexyl, PhCH2 2a-d) in 70-84% yield. According to 31P NMR and GLC data, a marked deviation from the statistical diastereomer ratio was obsd. only in the case of phosphonochloridate 2d.
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
277. Preparation and lipase-catalyzed optical resolution of 2,2,2-trifluoro-1-(naphthyl)ethanols
By Kato, Katsuya; Katayama, Masato; Gautam, Rakesh K.; Fujil, Shozo; Kimoto, Hiroshi
From Bioscience, Biotechnology, and Biochemistry (1995), 59(2), 271-6. Language: English, Database: CAPLUS, DOI:10.1271/bbb.59.271
The optical resoln. of racemic 2,2,2-trifluoro-1-(naphthyl)ethanols (TFNEs) was achieved by lipase-catalyzed enantioselective acetylation with vinyl acetate in octane, S-acetates and R-alcs. being obtained. The introduction of a Me group into the naphthalene ring at the 4-position (2b) or the 2-position (2c) markedly decreased the reactivity, in particular for 2c. 2,2,2-Trifluoro-1-(1-naphthyl)ethanol (2a) behaved differently from the regioisomer, 2,2,2-trifluoro-1-(2- naphthyl)ethanol (2d): the enantioselectivity of lipases [LIP (Pseudomonas), PLC (Alcaligenes), and ALC (Achromobacter)] was high for 2a but low for 2d, whereas lipases [AK and PS (Pseudomonas)] exhibited higher selectivity for 2d than for 2a. Three other derivs., 2,2,2-trifluoro-1-(phenyl)ethanol, 2,2,2-trifluoro-1-(indol-3-yl)ethanol, and 1-(1-naphthyl)ethanol, were prepd., and their behaviors with respect to lipase-catalyzed acetylation were compared with 2a.
