
Reference_01_08_2014_165529
.pdf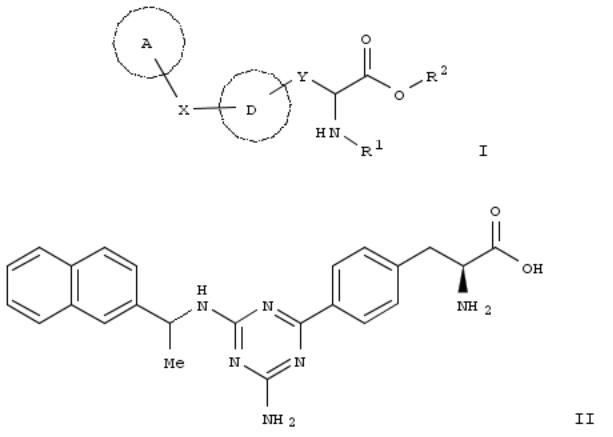
SciFinder® |
Page 121 |
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
164. Oxidation of alcohols to carbonyl compounds via alkoxysulfonium ylides: the Moffat, Swern, and related oxidations
By Tidwell, Thomas T.
From Organic Reactions (Hoboken, NJ, United States) (1990), 39, No pp. given. Language: English, Database: CAPLUS, DOI:10.1002/0471264180.or039.03
A review of the article Oxidn. of alcs. to carbonyl compds. via alkoxysulfonium ylides: the Moffat, Swern, and related oxidns.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
165. Simmons-Smith cyclopropanation reaction
By Charette, Andre B.; Beauchemin, Andre
From Organic Reactions (Hoboken, NJ, United States) (2001), 58, No pp. given. Language: English, Database: CAPLUS, DOI:10.1002/0471264180.or058.01
A review of the article Simmons-Smith cyclopropanation reaction.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
166. Preparation of pyrimidine derivatives as GPR119 G protein-coupled receptor modulators
By Fevig, John M.; Wacker, Dean A.
From PCT Int. Appl. (2008), WO 2008137435 A1 20081113, Language: English, Database: CAPLUS

SciFinder® |
Page 122 |
The title compds. with general formula I [wherein m = 1 or 2; n = 0 - 3; p and q = independently 0 - 2; A, B, and D = independently N, -CH, -C-halogen, -C-NH2, etc.; E = CH2, O, or NH, provided that when E is CH2 then at least one of A, B, or D is N; G = CH or N; Y is -NH, -N-alkyl, O, S, etc.; Z = absent or O; R1 = (un)substituted aryl or heteroaryl; R2 = (un)substituted cycloalkyl, aryl, heteroaryl, heterocyclyl, etc.] or enantiomers, diastereomers, prodrugs, pharmaceutically acceptable salts thereof were prepd. as GPR119 G protein-coupled receptor modulators useful in treating, preventing, or slowing the progression of diseases requiring GPR119 G protein-coupled receptor modulator therapy. For example, compd. II was prepd, in a multi-step synthesis. The in vitro GPR119 modulation activities of I was detd. in HIT-T15 cAMP assay, Human Tet-inducible cAMP assay, and Luciferase assay. (some data given). Formulations contg. I as actives ingredients were also disclosed in the present invention.
~13 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
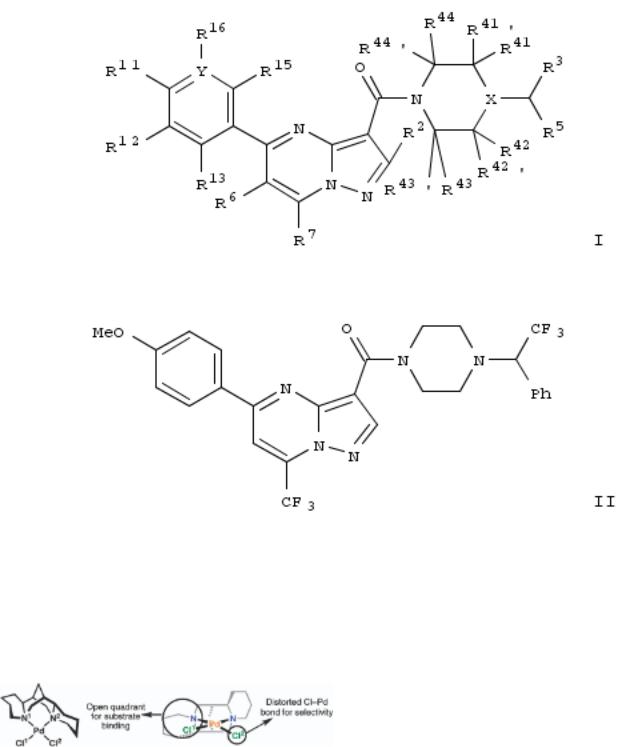
167. Alpha-substituted arylmethylpiperazine pyrazolo[1,5-a]pyrimidine amide derivatives as antiretroviral agents and their preparation and use in the treatment of HIV associated diseases
By Nitz, Theodore J.; Salzwedel, Karl; Finnegan, Catherine; Brunton, Shirley; Flanagan, Stuart; Montalbetti, Christian; Coulter, Thomas Stephen
From PCT Int. Appl. (2008), WO 2008134036 A1 20081106, Language: English, Database: CAPLUS
Derivs. of pyrazolopyrimidine compds. represented by formula I are disclosed. These pyrazolopyrimidine derivs. and pharmaceutical compns. comprising these derivs. are useful in the treatment of HIV mediated diseases and conditions. Compds. of formula I wherein R11 is H, OH, alkoxy, alkyl, cycloalkyl, dialkylamino, halo, etc.; R12, R13, R14 and R15 are independently is H, OH, alkoxy, alkyl and halo; R11R12 taken together to form heterocycle; Y is C and N; with proviso that if Y is N, then R14 is absent; R2 is H, halo, OH, CN, alkyl, alkoxy, alkoxyalkyl, etc.; R3 is alkyl, CN. haloalkyl, hydroxyalkyl,
alkoxyalkyl, etc. R41, R41', R43 and R43' are independently H, alkyl; R42, R42' and R44 and R44' are independently H, alkyl, cycloalkyl, hydroxyalkyl, etc.; R6 is H, halo, alkyl, alkoxy, alkoxyalkyl, etc.; R7 is H, alkyl, alkoxy, alkoxyalkyl,
alkylamidoalkyl, etc.; R13R6 may be taken together to form a cyclic ring; and pharmaceutically acceptable salts and solvates thereof, are claimed. Example compd. II was prepd. by reductive alkylation of piperazine with 2,2,2- trifluoroacetophenone; the resulting 1-(2,2,2-trifluoro-1-phenylethyl)piperazine TFA salt underwent amidation with 5-(4- methoxyphenyl)-6-methyl-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine-3-carbonyl chloride to give compd. II. All the invention compds. were evaluated for their antiretroviral activity. From the assay, it was detd. that compd. II exhibited IC50 value of less than 1.0 μM.
SciFinder® |
Page 123 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
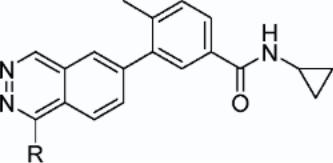
168. Structural Features and Reactivity of (Sparteine)PdCl2: A Model for Selectivity in the Oxidative Kinetic Resolution of Secondary Alcohols
By Trend, Raissa M.; Stoltz, Brian M.
From Journal of the American Chemical Society (2008), 130(47), 15957-15966. Language: English, Database: CAPLUS, DOI:10.1021/ja804955e
The chiral ligand (-)-sparteine and PdCl2 catalyze the enantioselective oxidn. of secondary alcs. to ketones and thus effect a kinetic resoln. The structural features of sparteine that led to the selectivity obsd. in the reaction were not clear. Substitution expts. with pyridine derivs. and structural studies of the complexes generated were carried out on (sparteine)PdCl2 and indicated that the C1 symmetry of (-)-sparteine is essential to the location of substitution at the metal center. Palladium alkoxides were synthesized from secondary alcs. that are relevant steric models for the kinetic resoln. The solid-state structures of the alkoxides also confirmed the results from the pyridine deriv. substitution studies. A model for enantioinduction was developed with C1 symmetry and Cl- as key features. Further studies of the diastereomers of (-)- sparteine, (-)-α-iso- and (+)-β-isosparteine, in the kinetic resoln. showed that these C2-sym. counterparts are inferior ligands in this stereoablative reaction.
~24 Citings
SciFinder® |
Page 124 |
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
169. Discovery of Highly Selective and Potent p38 Inhibitors Based on a Phthalazine Scaffold
By Herberich, Brad; Cao, Guo-Qiang; Chakrabarti, Partha P.; Falsey, James R.; Pettus, Liping; Rzasa, Robert M.; Reed, Anthony B.; Reichelt, Andreas; Sham, Kelvin; Thaman, Maya; et al
From Journal of Medicinal Chemistry (2008), 51(20), 6271-6279. Language: English, Database: CAPLUS, DOI:10.1021/jm8005417
Investigations into the structure-activity relationships (SAR) of a series of phthalazine-based inhibitors of p38 are described. These efforts originated from quinazoline 1 and through rational design led to the development of a series of orally bioavailable, potent, and selective inhibitors. Kinase selectivity was achieved by exploiting a collection of interactions with p38α including close contact to Ala157, occupation of the hydrophobic gatekeeper pocket, and a residue flip with Gly110. Substitutions on the phthalazine influenced the pharmacokinetic properties, of which compd. 16 displayed the most desirable profile. Oral dosing (0.03 mg/kg) of 16 in rats 1 h prior to LPS challenge gave a >50% decrease in TNFα prodn.
~32 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
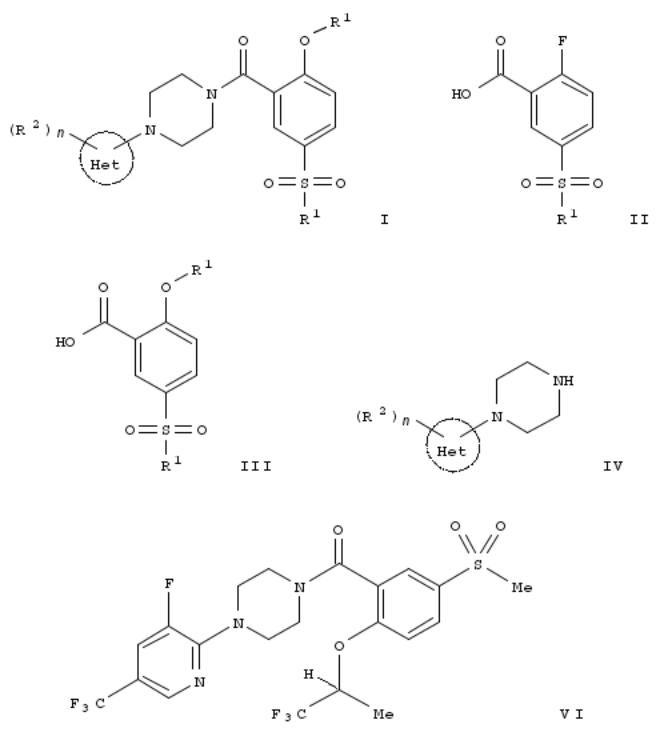
170. Preparation of N-benzoylpiperazine derivatives as glycine transporter 1 (GLYT-1) inhibitors
By Pfleger, Christophe; Waldmeier, Pius; Wang, Shaoning
From U.S. Pat. Appl. Publ. (2008), US 20080221327 A1 20080911, Language: English, Database: CAPLUS
The present invention relates to a process for prepn. of a compd. of formula [I; Het = a 6-membered heteroaryl contg. 1-3 nitrogen atoms; R1 = C1-6 alkyl, C3-6 cycloalkyl, NR4R5, halo-C1-6 alkyl; R2 = OH, halo, NO2, cyano, C1-6 alkyl, C3-6 cycloalkyl, halo-C1-6 alkyl, hydroxy-C1-6 alkyl, (CH2)o-C1-6 alkoxy, halo-C1-6 alkoxy, NR4R5, C(O)R6, SO2R7; R3 = C1-6 alkyl, C3-6 cycloalkyl, halo-C1-6 alkyl; R4, R5 = H, C1-6 alkyl; R6 = H, C1-6 alkyl, C1-6 alkoxy, NR4R5; R7 = C1-6 alkyl, halo- C1-6 alkyl, (CH2)o-C3-6 cycloalkyl, (CH2)o-C3-6 alkoxy, NR4R5; n = 0-3; o = 0-2] and pharmaceutically acceptable acid addn. salts thereof. The process comprises reacting a compd. of formula (II; R1 = same as above) with a compd. of formula R3OH (R3 = same as above) to obtain a compd. of formula (III; R1, R3 = same as above), and coupling the compd. of formula III in the presence of a coupling reagent or the corresponding acid halogenide with a compd. of formula (IV; R2, Het, n = same as above) to obtain the compd. of formula I. Thus, a colorless soln. of 700.0 g 2-fluoro-5- methanesulfonylbenzoic acid in 7.7 L N,N-dimethylacetamide was treated with 1,965.0 g cesium carbonate and 522.8
(S)-trifluoroisopropanol, warmed to 120°, and stirred under argon for 72 h to give, after workup, 84.0 % (S)-5- (methanesulfonyl)-2-(2,2,2-trifluoro-1-methylethoxy)benzoic acid (V) (840.0 g). A suspension of 1.2 kg V and 50 mL DMF in 15 L toluene was treated at room temp. within 1 h with a soln. of 485.2 g oxalyl chloride in 650 mL toluene and the suspension was stirred for 1 h at room temp. and dropped at room temp. within 30 to 45 min to a soln. of 1.0 kg 1-(3- fluoro-5-trifluoromethylpyridin-2-yl)piperazine in 12 L toluene and 1.1 L Et3N. The reaction mixt. was stirred at room temp. for 30 min to give, after workup, (S)-[4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-(methanesulfonyl)-2- (2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (VI).
SciFinder® |
Page 125 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
171. Bis(2,2,2-trifluoroethyl) Chlorophosphate
By Spinelli, Domenico; Petrillo, Giovanni
From e-EROS Encyclopedia of Reagents for Organic Synthesis (2001), No pp. given. Language: English, Database: CAPLUS, DOI:10.1002/047084289X.rn00589
A review of the article Bis(2,2,2-trifluoroethyl) Chlorophosphate.
~0 Citings
SciFinder® |
Page 126 |
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
172. Nonafluorobutanesulfonyl Fluoride
By Subramanian, Lakshminarayanapuram R.; Martinez, Antonio Garcia; Hanack, Michael
From e-EROS Encyclopedia of Reagents for Organic Synthesis (2001), No pp. given. Language: English, Database: CAPLUS, DOI:10.1002/047084289X.rn061
A review of the article Nonafluorobutanesulfonyl Fluoride.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
173. (S)-(-)-a-Methoxy-a-(trifluoromethyl)phenylacetic Acid
By Jaen, Juan C.
From e-EROS Encyclopedia of Reagents for Organic Synthesis (2001), No pp. given. Language: English, Database: CAPLUS, DOI:10.1002/047084289X.rm126
A review of the article (S)-(-)-a-Methoxy-a-(trifluoromethyl)phenylacetic Acid.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
174. Graftable chiral ligands for surface organometallic materials: calixarenes bearing asymmetric centers directly attached to the lower rim
By Solovyov, Andrew; Notestein, Justin M.; Durkin, Kathleen A.; Katz, Alexander
From New Journal of Chemistry (2008), 32(8), 1314-1325. Language: English, Database: CAPLUS, DOI:10.1039/b801434p
A family of chiral lower-rim substituted calixarene ligands 1-14 is synthesized and characterized as prospective ligands for synthesis of asym. active sites for heterogeneous catalysis and adsorption using a surface organometallic approach. The ligands possess asym. centers directly attached to lower-rim oxygens; the asym. centers are tailored with sterically bulky naphthyl and electron withdrawing CF3 substituents. 1H and 13C NMR spectroscopies demonstrate that monoand 1,3-di-alkylated calixarenes synthesized using this procedure adopt the cone-shaped conformation, which is stabilized by multiple hydrogen bonds at the lower rim. Smaller pendant groups and polar solvents increase the dialkylation/monoalkylation ratio. The reaction proceeds with full inversion of configuration as established via single crystal X-ray diffraction of an N-Cbz-protected aminocyclopentoxy-modified calixarene. The calixarenes synthesized by this approach are characterized using CD (CD) spectroscopy and ab initio modeling, which is used to identify the minimal mol. fragment responsible for the obsd. lowest energy Cotton effect in the CD spectrum. Factors that influence the intramol. induction of asymmetry throughout the calixarene scaffold are investigated by systematically varying substituent steric bulk, connectivity of the asym. center to the calixarene core, and the extent of hydrogen bonding at the calixarene lower rim. The asymmetry of the calixarene core is quantified using 1H NMR spectroscopic shifts of arom. meta and methylene bridge hydrogens and supported using ab initio calcns. The results demonstrate that calixarene core asymmetry is most strongly induced for rigid calixarene cores-an attribute that is expected to be preserved upon metal complexation and anchoring of these prospective ligands.
~5 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
175. Synthesis and utilization of trifluoromethylated amino alcohol ligands for the enantioselective Reformatsky reaction and addition of diethylzinc to N-(diphenylphosphinoyl)imine
By Xu, Xiu-Hua; Qiu, Xiao-Long; Qing, Feng-Ling
From Tetrahedron (2008), 64(30-31), 7353-7361. Language: English, Database: CAPLUS,
DOI:10.1016/j.tet.2008.05.043
Trifluoromethylated amino alc. ligands, which were designed and conveniently prepd., were successfully applied in the enantioselective Reformatskii reaction and addn. of diethylzinc to N-(diphenylphosphinoyl)imine, resp. The influence of the substituents on C-3 position and the amino moiety on the enantioselectivity was carefully studied. In the best cases, ligand (2S,3S)-3-(diethylamino)-1,1,1-trifluoro-2-butanol exhibited good selectivity for the enantioselective Reformatskii reaction in 86% ee and ligand (2S,3S)-3-benzylamino-1,1,1-trifluoro-4,4-dimethyl-2-pentanol provided excellent enantioselectivity in the addn. of diethylzinc to N-(diphenylphosphinoyl)imine with 95% ee.
SciFinder® |
Page 127 |
~8 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
176. Modulation of Peripheral Serotonin Levels by Novel Tryptophan Hydroxylase Inhibitors for the Potential Treatment of Functional Gastrointestinal Disorders
By Shi, Zhi-Cai; Devasagayaraj, Arokiasamy; Gu, Kunjian; Jin, Haihong; Marinelli, Brett; Samala, Lakshman; Scott, Sheldon; Stouch, Terry; Tunoori, Ashok; Wang, Ying; et al
From Journal of Medicinal Chemistry (2008), 51(13), 3684-3687. Language: English, Database: CAPLUS, DOI:10.1021/jm800338j
The discovery of a novel class of peripheral tryptophan hydroxylase (TPH) inhibitors is described. This class of TPH inhibitors exhibits excellent potency in in vitro biochem. and cell-based assays, and it selectively reduces serotonin levels in the murine intestine after oral administration without affecting levels in the brain. These TPH1 inhibitors may provide novel treatments for gastrointestinal disorders assocd. with dysregulation of the serotonergic system, such as chemotherapy-induced emesis and irritable bowel syndrome.
~22 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
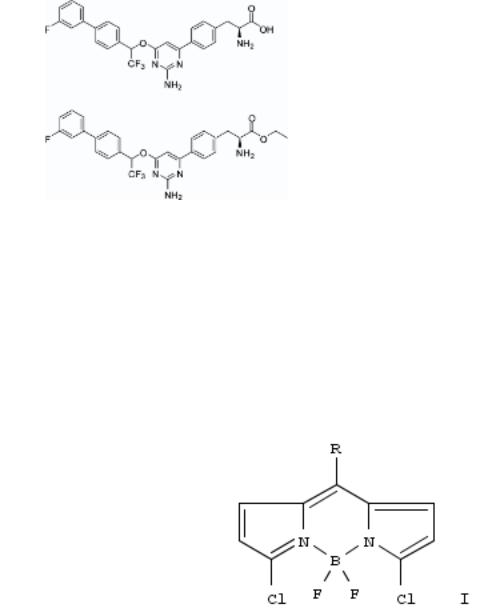
177. Functionalization of the 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) core
By Li, Lingling; Nguyen, Binh; Burgess, Kevin
From Bioorganic & Medicinal Chemistry Letters (2008), 18(10), 3112-3116. Language: English, Database: CAPLUS, DOI:10.1016/j.bmcl.2007.10.103
The new BODIPY systems I [R = 4-BrC6H4, CF3] were prepd. and then used as substrates to explore SNAr and F-B displacement reactions. Chloride was easily displaced from I [R = 4-BrC6H4] by a piperidinecarboylate, methylmagnesium bromide selectively displaced fluoride, and cyanide could attack both sites. I [R = CF3] readily added soft nucleophiles to the electrophilic carbon atoms, providing a new method for bioconjugation of BODIPYs to proteins while also introducing a 19F probe.
~59 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
178. Probing the hydrophobic pocket of the active site in the particulate methane monooxygenase (pMMO) from Methylococcus capsulatus (Bath) by variable stereoselective alkane hydroxylation and olefin epoxidation
By Ng, Kok-Yaoh; Tu, Li-Chun; Wang, Yane-Shih; Chan, Sunney I.; Yu, Steve S.-F.
From ChemBioChem (2008), 9(7), 1116-1123. Language: English, Database: CAPLUS, DOI:10.1002/cbic.200700628
SciFinder® |
Page 128 |
PMMO from M. capsulatus (Bath) oxidizes straight-chain C1-C5 alkanes and alkenes to form their corresponding 2-alcs. and epoxides. According to expts. performed with cryptically chiral ethane and D,L-[2-2H1,3-2H1]butane, the reactions proceed through the concerted O-atom insertion mechanism. However, when propene and but-1-ene are used as epoxidn. substrates, the enantiomeric excesses (ees) of the enzymic products are only 18 and 37%, resp. This relatively poor stereoselectivity in the enzymic epoxidn. presumably reflects low stereochem. differentiation between the re and si faces in the hydrophobic pocket of the active site. Further insights into the reaction mechanism are now provided by studies on trans-but-2-ene, which reveal only the D,L-2,3-dimethyloxirane products, and on cis-but-2-ene, which yield only the meso product. These observations indicate that the enzymic epoxidn. indeed proceeds through electrophilic syn addn. To achieve better facial selectivity, the authors have also used 3,3,3-trifluoroprop-1-ene as the substrate. The products obtained are 90% (2S)-oxirane. When 1,1,1-trifluoropropane is the substrate, the hydroxylation at the 2-carbon exhibits an inverse chiral selectivity relative to that seen with normal butane, if the authors consider the size of the CF3 group in the fluorinated propane to be comparable to one of the Et groups in butane. These expts. are beginning to delineate the factors that influence the orientations of various substrates in the hydrophobic cavity of the active site in the enzyme.
~14 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
179. Preparation of diaryloxadiazole derivatives for use as antiinflammatory and immunosuppressive agents
By Albert, Rainer; Cooke, Nigel Graham; Lewis, Ian; Weiler, Sven; Zecri, Frederic
From PCT Int. Appl. (2008), WO 2008037476 A1 20080403, Language: English, Database: CAPLUS
Title compds. I [R1 = substituted Ph, pyridinyl, pyranyl, biphenylyl, etc.; R2 = SO2NH2, SO2NHR11CO2H, R7NR8R9, or (un)substituted heterocycle; R3 and R4 = H; or one = H or alkyl, while the other = alkyl or haloalkoxy; R7 = cycloalkylene or substituted alkylene; R8 and R9 independently = H, (un)substituted alkyl, R10CO, or (un)substituted heterocycle; or together with the N which they are attached form (un)substituted heterocycle; R10 = alkyl, cycloalkyl, Ph, or phenylalkyl; R11 = alkylene optionally interrupted by O, S, or C=CH2; with several provisions], and their pharmaceutically acceptable salts, are prepd. and disclosed as antiinflammatory and immunosuppressive agents. Thus, e.g., II was prepd. by Suzuki coupling of 4-chloro-3-trifluoromethylbenzoic acid with phenylboronic acid followed by heterocyclization with N-hydroxy-4- sulfamoyl-3-trifluoromethoxybenzamidine (prepn. given). I were evaluated in S1P1 GTP [γ-35S] binding assays, e.g., III demonstrated an EC50 value of < 100 (nM).

SciFinder® |
Page 129 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
180. Phthalazine, azaand diaza-phthalazine compounds as protein kinase inhibitors and their preparation, pharmaceutical compositions and use in the treatment of protein kinase-mediated diseases
By Tasker, Andrew; Zhang, Dawei; Pettus, Liping H.; Rzasa, Robert M.; Sham, Kelvin K. C.; Xu, Shimin; Chakrabarti, Partha Pratim
From PCT Int. Appl. (2008), WO 2008030466 A1 20080313, Language: English, Database: CAPLUS
The invention comprises a class of compds. of formula I useful for the prophylaxis and treatment of protein kinase mediated diseases, including inflammation and related conditions. The invention also comprises pharmaceutical compns. including one or more compds. of formula I, uses of such compds. and compns. for treatment of kinase mediated diseases including rheumatoid arthritis, psoriasis and other inflammation disorders, as well as intermediates and processes useful for the prepn. of compds. of formula I. Compds. of formula I wherein each of A1, A2, A3, A4, A5 and A6 is CR3 and N, provided that no more than two of A3, A4, A5 and A6 is N; L1 is CONH and derivs., NHCO and derivs., NHCONH and derivs. and SO2NH and derivs.; R1 is (un)substituted C1-12 alkyl, (un)substituted C0-2 alkyl-C3-6 cycloalkyl, (un)substituted C0-2 alkyloxy-C1-10 alkyl, and (un)substituted C0-2 alkylthio-C1-10 alkyl; R2, each R3 and R5 are independently H, halo, haloalkyl, NO2, CN, C1-10 alkyl, etc.; R4 is (un)substituted (un)satd. 3- to 8-membered (hetero)cyclic ring and (un)substituted (un)satd. bi(hetero)cyclic ring; n is 0, 1, 2 and 3; and their stereoisomers, pharmaceutically acceptable salts thereof, are claimed. Example compd. II was prepd. by amination of 6-bromo-1- chlorophthalazine with isopropylamine; the resulting 6-bromo-N-isopropylphthalazin-1-amine underwent cross-coupling with 4-methyl-N-(1-ethyl-1H-pyrazol-5-yl)-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide to give compd. II. All the invention compds. were evaluated for their protein kinase inhibitory activity (some data given).

SciFinder® |
Page 130 |
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
181. Preparation of fluorine-containing carbonyl compounds by oxidation of fluorine-containing alcohols with zirconiasupported metal oxide catalysts
By Mimura, Hideyuki; Watabe, Akio; Kawada, Kosuke
From Jpn. Kokai Tokkyo Koho (2008), JP 2008029905 A 20080214, Language: Japanese, Database: CAPLUS
X(CF2)nCOR (X = H, F; R = H, C1-10 alkyl, C6-20 aryl; n = 1-10; excluding CF3CHO) and/or X(CF2)nCR(OH)2 [X, R, n = same as above; excluding CF3CH(OH)2] are prepd. by gas-phase oxidn. of X(CF2)nCHROH (X, R, n = same as above;
excluding CF3CH2OH) with O or O-contg. gases in the presence of ZrO2-supported metal oxides contg. V oxides. Thus, H(CF2)2CH2OH (I) and air were passed through a reactor filled with ZrO2-supported tin vanadium oxide at 280° for 1 h to give H(CF2)2CH(OH)2 with selectivity 78% and conversion of I 96%.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
182. Substituted 3-amino-pyrrolidine-4-lactam derivatives, processes for preparing them, pharmaceutical compositions containing them, and their use as DPP-IV (dipeptidyl peptidase IV) inhibitors
By Benbow, John William; Piotrowski, David Walter; Hui, Yu
From PCT Int. Appl. (2007), WO 2007148185 A2 20071227, Language: English, Database: CAPLUS
