
Reference_01_08_2014_165529
.pdf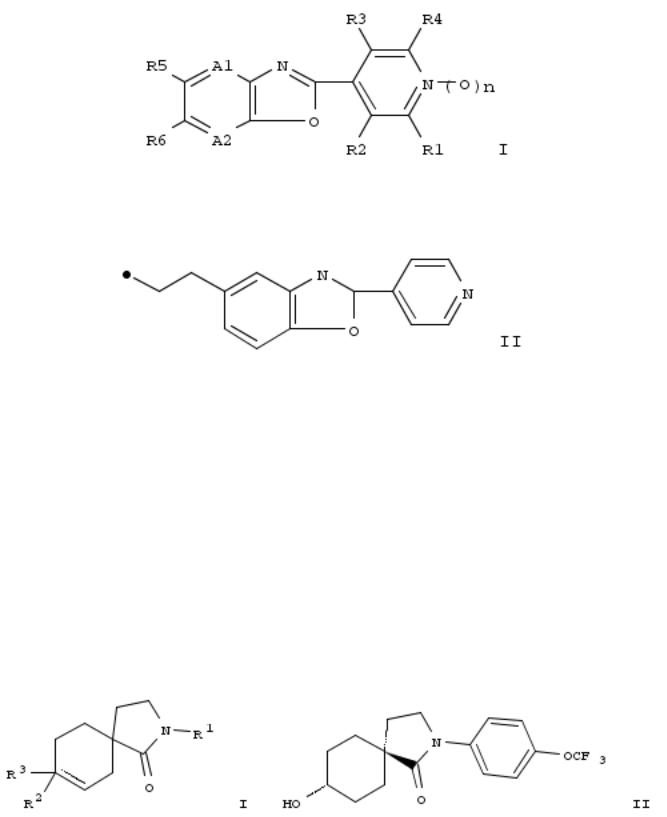
SciFinder® |
Page 71 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
91. Preparation of spiro-condensed cyclohexane derivatives as hormone sensitive lipase inhibitors useful for the treatment of diabetes
By Ackermann, Jean; Brugger, Stephan; Conte, Aurelia; Hunziker, Daniel; Neidhart, Werner; Nettekoven, Matthias; Schulz-Gasch, Tanja; Wertheimer, Stanley
From PCT Int. Appl. (2011), WO 2011045292 A1 20110421, Language: English, Database: CAPLUS
The invention relates to compds. of formula I as well as pharmaceutically acceptable salts thereof that can be used in the form of pharmaceutical compns. as hormone sensitive lipase (HSL) inhibitors for the treatment of diabetes. Compds. of formula I wherein dashed bond is single and double bond; R1 is alkyl, Ph, phenylalkyl, pyridinyl, etc.; R2 is H, alkyl, cycloalkyl, hydroxyalkyl, etc.; R3 is R4A; when dashed bond is double bond, the R3 is absent; when dashed bond is single bond, then R2R3 can be taken together to form =O; A is O, NH and derivs., S, SO, SO2, etc.; R4 is H, alkyl, cycloalkyl, hydroxyalkyl, etc.; and pharmaceutically acceptable salts thereof, are claimed. Example compd. II was prepd. by acetalization of 4-oxocyclohexanecarboxylic acid Et ester; the resulting 1,4-dioxaspiro[4.5]decane-8-carboxylic acid Et ester underwent alkylation with 2-bromoethyl Me ether followed by hydrolysis to give 1-(2-methoxyethyl)-4- oxocyclohexanecarboxylic acid Et ester, which underwent hydride redn. to give trans-4-hydroxy-1-(2- methoxyethyl)cyclohexanecarboxylic acid Et ester, which underwent cyclocondensation with 4-trifluoromethoxyaniline to give compd. II. All the invention compds. were evaluated for their HSL inhibitory activity. From the assay, it was detd. that compd. II exhibited IC50 value of 0.47 μM.
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
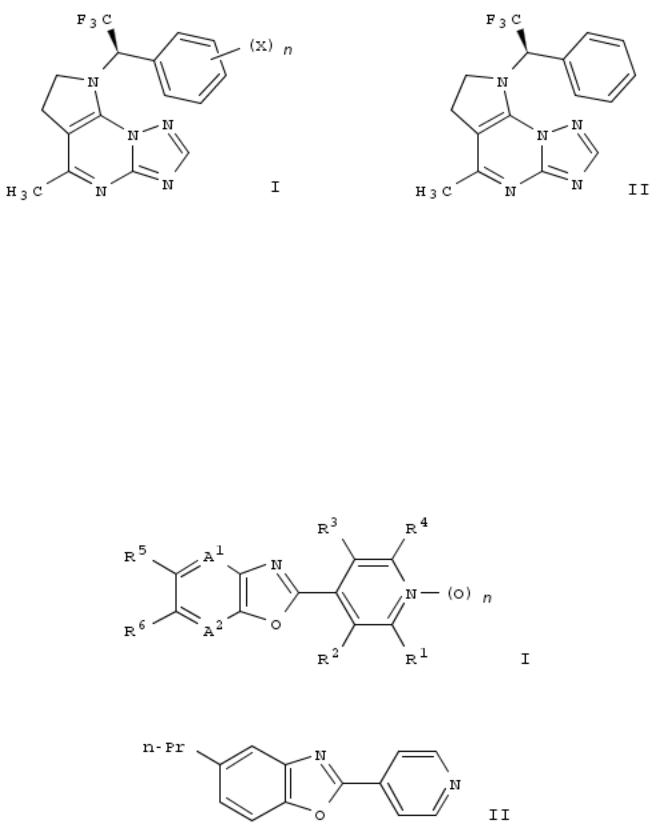
92. Pyrrolo[3,2-e][1,2,4]triazolo[1,5-a]pyrimidine derivatives as inhibitors of microglia activation and their preparation and use for the treatment of diseases
SciFinder® |
Page 72 |
By Scopes, David
From PCT Int. Appl. (2011), WO 2011042496 A1 20110414, Language: English, Database: CAPLUS
The invention relates to pyrrolo[3,2-e][1,2,4]triazolo[1,5-a]pyrimidine derivs. of formula I, which are inhibitors of microglia activation and which are useful in the treatment of diseases caused by activation of microglia, particularly Alzheimer's disease. Compds. of formula I wherein each X is independently F and Cl; n is 0 to 2; and pharmaceutically acceptable salts thereof, are claimed. Example compd. II was prepd. by a multistep procedure (procedure given). All the invention compds. were evaluated for their ability to inhibit microglia activation (data given).
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
93. Preparation of 2-(pyridin-4-yl)benzoxazole derivatives and their composition and method for controlling arthropod pests
By Otsuki, Junko
From PCT Int. Appl. (2011), WO 2011040629 A1 20110407, Language: English, Database: CAPLUS
The invention provides an arthropod pests control compn. comprising, as active ingredients, a condensed heterocyclic compd. of formula I and a neonicotinoid compd.; a method for controlling arthropod pests which comprises applying effective amts. of a condensed heterocyclic compd. and a neonicotinoid compd. to the arthropod pests or a locus where the arthropod pests inhabit; and so on. Compds. of formula I wherein A1 and A2 are independently N and CR7; R1 and R4 are independently halo and H; R2 and R3 are independently (un)substituted C1-6 acyclic hydrocarbon, (un)substituted C3-6 alicyclic hydrocarbon, (un)substituted Ph, etc.; R5 and R6 are independently (un)substituted C1-6 acyclic hydrocarbon, (un)substituted C3-6 alicyclic hydrocarbon, halo, H, etc.; R5R6 may be taken together to form (un)substituted 5- to 6-membered ring; R7 is (un)substituted C1-3 alkyl, (un)substituted C1-3 alkoxy, CN, halo and H; n is 0 and 1; are claimed. Example compd. II was prepd. by cyclocondensation of isonicotinic acid with 2-amino-4- propylphenol. All the invention compds. were evaluated for their pesticidal activity (some data given).
SciFinder® |
Page 73 |
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
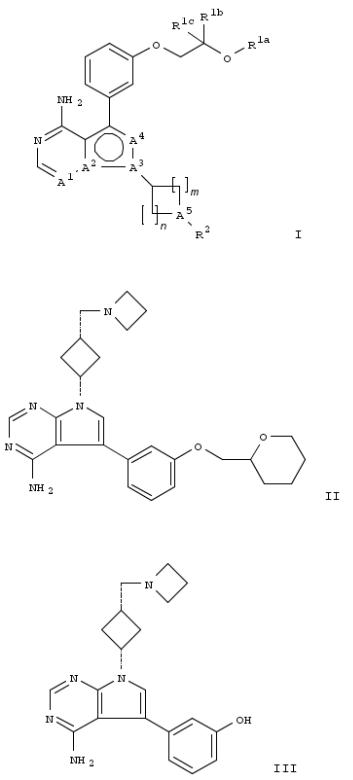
94. Preparation of ether derivatives of bicyclic heteroaryls as inhibitors of IGF-1R tyrosine kinase activity for treatment of proliferative diseases
By Chen, Bei; Fairhurst, Robin Alec; Floersheimer, Andreas; Furet, Pascal; Guagnano, Vito; Jiang, Songchun; Lu, Wenshuo; Marsilje, Thomas H.; McCarthy, Clive; Michellys, Pierre-Yves; et al
From PCT Int. Appl. (2011), WO 2011029915 A1 20110317, Language: English, Database: CAPLUS
The invention relates to compds. of formula I (wherein A1-A4 represent N or a C, with no two adjacent A's being N or C; R1a is branched C3-8alkyl or C3-10cycloalkyl; R1b and R1c are independently hydrogen or C1-7alkyl; or R1a and R1b can form part of ring; or R1a-R1c can form part of a ring; m = 1 or 2; n = 1 or 2; R2 is OH, SH, 3-12-membered monocyclic or bicyclic heterocyclyl); to processes for the prepn. of such compds.; pharmaceutical compns. comprising such compds.; such compds. as a medicament; such compds. for the treatment of a proliferative disease. The compds. of formula I are potent inhibitors of the tyrosine kinase activity of the insulin like growth factor I receptor (IGF-IR) and inhibit IGF-1R- dependent cell proliferation. Example compd. II, prepd. by reacting intermediate III and rac-2-tetrahydropyranylmethanol, had an IC50 of 119-139 nM in a cellular assay that measured inhibition of IGFR1 phosphorylation in Hek292 cells transduced with the receptor.
SciFinder® |
Page 74 |
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
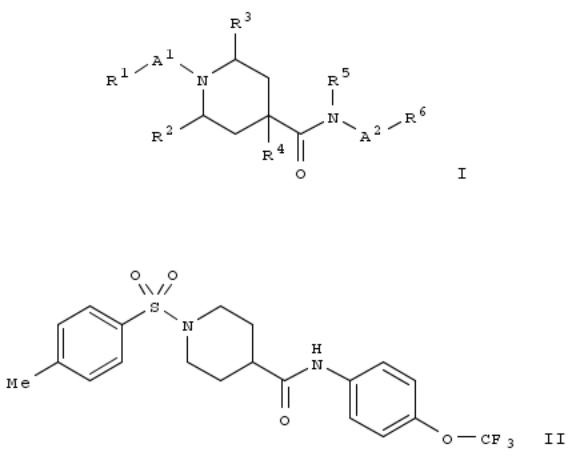
95. Preparation of azacyclic derivatives as hormone sensitive lipase (HSL) inhibitors useful in treatment of diabetes
By Ackermann, Jean; Conte, Aurelia; Neidhart, Werner; Nettekoven, Matthias; Wertheimer, Stanley From PCT Int. Appl. (2011), WO 2011029808 A1 20110317, Language: English, Database: CAPLUS
SciFinder® |
Page 75 |
Title compds. I [R1 = dimethylpropyl, dimethylbutyl, cyclopropylalkyl, pyrazolyl, methyltrifluoromethyl-1H-pyrazolyl, morpholinyl, Ph, 2-chlorophenyl, 4-methylphenyl or 4-methoxyphenyl; R2 = H, alkyl, hydroxyalkyl or alkoxyalkyl; R3 = H, alkyl, hydroxyalkyl or alkoxyalkyl; R4 = H, halo, alkyl, hydroxyalkyl or alkoxyalkyl; R5 = H or alkyl; R6 = 2,3- dihydrobenzofuranyl, alkylpyridin-3-yl, haloalkoxypyridin-3-yl, pyridazinyl, alkoxypyridazinyl, alkyltrifluoromethyl-1H- pyrazolyl, (un)substituted Ph; A1 = carbonyl or S(O)2; A2 = single bond, CH2CH2 or G; G = 3- to 8-membered ring], and their pharmaceutically acceptable salts, are prepd. and disclosed. Thus, e.g., II was prepd. by amidation of 1-[(4- methylphenyl)sulfonyl]-4-piperidinecarboxylic acid with 4-trifluoromethoxyaniline. II exhibited IC50 value of 0.08 μM in human HSL inhibition assay. The compds. of the invention are useful for the treatment or prophylaxis of illnesses, esp. in the treatment or prophylaxis of diabetes, metabolic syndrome, dyslipidemia, atherosclerosis or obesity.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
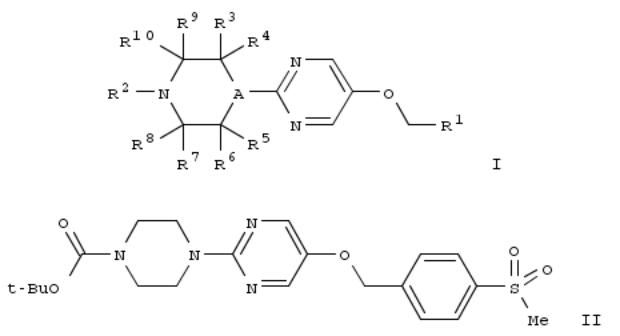
96. 4-(Pyrimidin-2-yl)piperazine and 4-(pyrimidin-2-yl)piperidine derivatives as GPR119 modulators and their preparation and use in the treatment of obesity and diabetes mellitus
By Birch, Alan Martin; Broo, Dan Anders; Butlin, Roger John; Clarke, David Stephen; Davidsson, Ojvind Percy; De La Motte, Hanna; Johansson, Kjell Erik; Leach, Andrew; Macfaul, Philip Alexander; O'Donnell, Charles John; et al From PCT Int. Appl. (2011), WO 2011030139 A1 20110317, Language: English, Database: CAPLUS
A compd. of formula I or a pharmaceutically acceptable salt thereof, processes for prepg. such compds., their use as GPR119 modulators, methods for their therapeutic use, particularly in the treatment of obesity and diabetes mellitus, and pharmaceutical compns. contg. them. Compds. of formula I wherein A is CH and N; R1 is substituted Ph, substituted pyridinyl, (un)substituted pyrimidinyl, etc.; R2 is CO2Rx, (un)substituted pyrimidinyl, (un)substituted oxazolidinyl; Rx is (un)substituted C1-6 alkyl, (un)substituted C3-6 cycloalkyl, satd. cyclic ether, etc.; R3 - R10 are independently H and (un)substituted C1-4 alkyl; R3R7, R3R5 or R7R9 taken together to form methylene or ethylene bridge; and pharmaceutically acceptable salts thereof, are claimed. Example compd. II was prepd. by O-alkylation of tert-Bu 4-(5- hydroxypyrimidin-2-yl)piperazine-1-carboxylate with 1-bromomethyl-4-methylsulfonylbenzene. All the invention compds. were evaluated for their GPR119 modulatory activity. From the assay, it was detd. that compd. II exhibited 83 % activation at 30 μM concn.
SciFinder® |
Page 76 |
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
97. Treasures from the Free Radical Renaissance Period - Miscellaneous hexenyl radical kinetic data
By Beckwith, Athelstan L. J.; Schiesser, Carl H.
From Organic & Biomolecular Chemistry (2011), 9(6), 1736-1743. Language: English, Database: CAPLUS, DOI:10.1039/c0ob00708k
Rate const. data and Arrhenius parameters were detd. for substituted hexenyl radicals of differing electronic and steric demand. Electron-withdrawing groups (CF3, CO2Et) directly attached to the radical center slightly accelerate 5-exo ring-
closure (kcis + ktrans 2.1 × 105 s-1 at 25°) relative to donating groups (OMe; 1.6 × 105 s-1 at 25°). Sterically demanding groups (tert-Bu), as expected, slow the cyclization process (1 × 105 s-1). These observations are consistent with subtle
changes in activation energy for 5-exo ring-closure. The nature of the solvent would appear to have a significant influence on this chem. with the cis/trans stereoselectivity sometimes improved as the solvent polarity is increased. Except for the system contg. the CF3 (electron-withdrawing) group which displays an increase in the cyclization/capture rate const. (kc/kH), a general decrease in the kc/kH ratio as solvent polarity is increased is noted; these changes were speculated to arise mainly from changes in kH in the various solvents employed.
~12 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
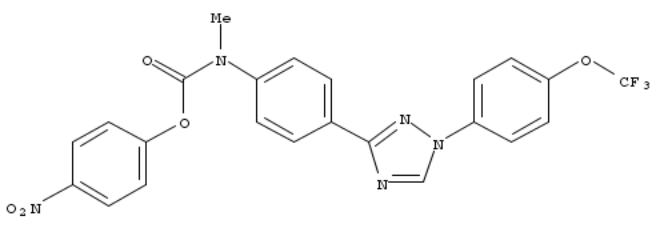
98. Pesticidal compositions of prepared heteroaryl-N-aryl carbamates and their use in pest control
By Lambert, William; Crouse, Gary; Sparks, Thomas; Cudworth, Denise
From PCT Int. Appl. (2011), WO 2011017513 A1 20110210, Language: English, Database: CAPLUS
The present invention concerns novel heteroaryl-N-aryl carbamates and their use in pest control, as insecticides and acaricides. This invention also includes prepn. of the pesticide compns. contg. the compds., and methods of controlling insects using the compds. Title compds. were prepd. and tested for control of beet armyworm (Spodoptera exigua), corn earworm (Helicoverpa zea) and green peach aphid (Myzus persicae).
SciFinder® |
Page 77 |
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
99. Preparation of fused imidazole derivatives having TTK kinase inhibitory activity
By Kusakabe, Ken-Ichi; Yoshida, Hiroshi; Nozu, Kohei; Hashizume, Hiroshi; Tadano, Genta; Sato, Jun; Tamura, Yuusuke; Mitsuoka, Yasunori
From PCT Int. Appl. (2011), WO 2011013729 A1 20110203, Language: Japanese, Database: CAPLUS
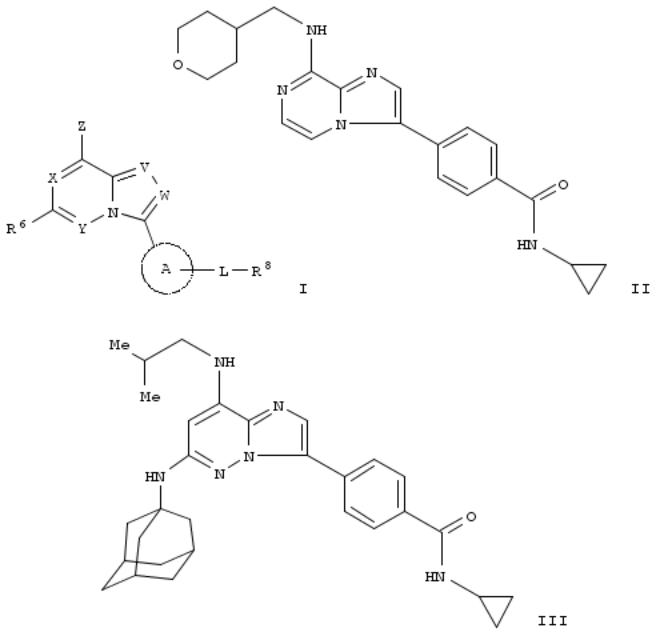
There are provided fused imidazole compds. represented by general formula [I; (X, Y, V, W) = (N:, :C(R1), :N, C(R7):), (CR2:, :N, :N, C(R7):), (N:, :N, :N, C(R7):), (N:, :C(R1 ), :N, N:), or (N:, :C(R1 ), O, N:); R1 , R2 = H, halo, HO, cyano, NO2, CO2H, each (un)substituted alkyl, alkenyl, or alkynyl; Z = NR3R4, OR5; R3 = H, (un)substituted alkyl; R4, R5 = H, each (un)substituted alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, aryl, cycloalkenyl, aryl, heteroaryl, heterocyclyl, alkoxy, or alkylsulfonyl; R6 = H, halo, HO, cyano, each (un)substituted alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, aryl, heteroaryl, heterocyclyl, NH2, acyl, alkoxy, aryloxy, heteroaryloxy, cycloalkyloxy, heterocyclyloxy, or carbamoyl, etc.; R7 = H, halo, HO, cyano, NO2, CO2H, each (un)substituted alkyl, alkenyl, or alkynyl; ring A = each (un)substituted carbocyclic, arom. heterocyclic, nonarom. carbocyclic, or nonarom. heterocyclic ring; L = a single bond, C(O), NRA, NR8C(O), S(O)n, NRCS(O)n, or each (un)substituted alkylene, alkenylene, or alkynylene; R8 = H, halo, HO, cyano, CO2H, each (un)substituted alkoxy, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, aryl, heteroaryl, heterocyclyl, or NH2; RA, RB, RC, RD = H, (un)substituted alkyl; or R8 and RA or R8 and RC together with the adjacent N atom from (un)substituted N-contg. heterocyclic ring; n = 0,1], pharmaceutically acceptable salts thereof, or solvates thereof. These compds. such as imidazo[1,2-a]pyrazines, imidazo[1,2-b]pyridazines, and isooxazolo[4,5-d]pyrimidines have a TTK inhibitory action and are useful for the prevention and/or treatment of cancer or as immunosuppressants for the treatment of immune diseases or autoimmune diseases. Thus, a soln. of 150 mg N-cyclopropyl-4-(8-(methylsulfonyl)imidazo[1,2-a]pyrazin-3- yl)benzamide in 1 mL dioxane was treated with 96.7 mg (tetrahydro-2H-pyran-4-yl)methanamine and refluxed for 12 h to give N-cyclopropyl-4-(8-[[(tetrahydro-2H-pyran-4-yl)methyl]amino]imidazo[1,2-a]pyrazin-3-yl)benzamide (II). II and compd. (III) in vitro showed IC50 of 0.0347 and 0.0019 μM against TTK kinase, resp., and IC50 of 1.0< to ≤10 and ≤0.05 μM, resp., against human non small cell lung cancer (A549) cells.
SciFinder® |
Page 78 |
~10 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
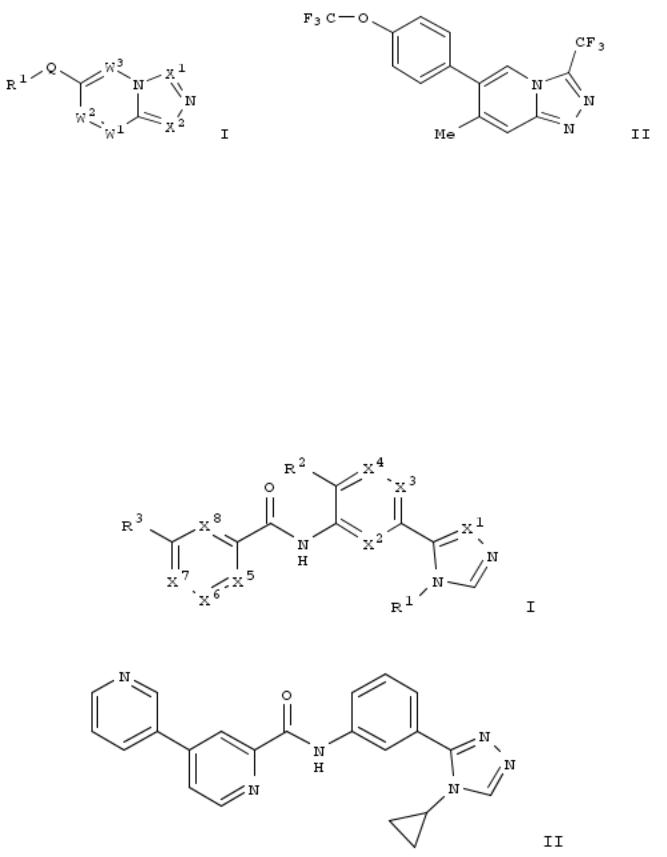
100. Preparation of [1,2,4]triazolo[4,3-a]pyridine derivatives and analogous as sodium channel modulators
By Corkey, Britton; Elzein, Elfatih; Jiang, Robert; Kalla, Rao; Kobayashi, Tetsuya; Koltun, Dmitry; Li, Xiaofen; Notte, Gregory; Parkhill, Eric; Perry, Thao; et al
From U.S. Pat. Appl. Publ. (2011), US 20110021521 A1 20110127, Language: English, Database: CAPLUS
The invention relates to compds. that are sodium channel inhibitors and to their use in the treatment of various disease states, including cardiovascular diseases and diabetes. Title compds. I [R1 = (un)substituted aryl or heteroaryl; W1 = N or CR2, wherein R2 independently = H, amino, CF3, etc.; W3 = N or CR4, wherein R4 independently = H, hydroxy, halo, alkyl, alkoxy, etc.; Q = covalent bond or alkynylene; X1 = N or CRa, wherein Ra = H, (un)substituted alkyl, alkoxy, etc.; Y = covalent bond or (un)substituted alkylene; X2 = N or CRb, wherein Rb = H, CF3, OCF3, etc.], and their pharmaceutically acceptable salts, are prepd. and disclosed. Thus, e.g., II was prepd. (general procedure given). The compds. of the invention were tested in the late sodium assay, II showed late INa inhibition of 49% at 1 μM concn. and 67.5% at 10 μM.
SciFinder® |
Page 79 |
~2 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
101. N-(Azolylaryl) benzamides and pyridinecarboxamides as apoptosis signal-regulating kinase inhibitors and their preparation and use in the treatment of ASK1-mediated diseases
By Corkey, Britton; Graupe, Michael; Koch, Keith; Melvin, Lawrence S., Jr.; Notte, Gregory
From U.S. Pat. Appl. Publ. (2011), US 20110009410 A1 20110113, Language: English, Database: CAPLUS
The invention related to compds. of formula I have apoptosis signal-regulating kinase (ASK1) inhibitory activity, and are thus useful in the treatment of ASK1-mediated conditions, including autoimmune disorders, inflammatory diseases, cardiovascular diseases and neurodegenerative diseases. The invention also relates to pharmaceutical compns. comprising one or more of the compds. of formula I, and to methods of prepg. the compds. of formula I. Compds. of formula I wherein R1 is C1-10 alkyl, C3-8 cycloalkyl, C1-10 alkenyl, C1-10 alkynyl, etc.; R2 is H, halo, CN, alkoxy and (un)substituted alkyl; R3 is (un)substituted aryl, (un)substituted heteroaryl and (un)substituted heterocyclyl; X1 - X8 are independently CR4 and N; R4 is H, OH, halo, etc.; X5X6 or X6X7 can be taken together to form fused cycloalkyl, aryl, etc.; with provisions; are claimed. Example compd. II was prepd. by cross-coupling of 4-bromo-N-(3-(4-cyclopropyl-4H-1,2,4- triazol-3-yl)phenyl)picolinamide with pyridine-3-boronic acid. All the invention compds. were evaluated or their ASK1 inhibitory activity (some data given).
~3 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 80 |
102. Chiral recognition ability of cellulose derivatives bearing pyridyl and bipyridyl residues as chiral stationary phases for high-performance liquid chromatography
By Katoh, Yasunaka; Tsujimoto, Yasutaka; Yamamoto, Chiyo; Ikai, Tomoyuki; Kamigaito, Masami; Okamoto, Yoshio From Polymer Journal (Tokyo, Japan) (2011), 43(1), 84-90. Language: English, Database: CAPLUS, DOI:10.1038/pj.2010.108
Cellulose derivs. bearing pyridyl and bipyridyl residues were synthesized, and their recognition abilities as chiral stationary phases for HPLC were evaluated. Compared with cellulose derivs. bearing these residues at the 2-, 3- and 6- positions of a glucose ring, the regioselectively substituted derivs. exhibited relatively high chiral recognition. The recognition ability of the derivs. was significantly influenced by the coordination of a Cu(II) ion to the bipyridyl residues. The derivs. were also used for ligand-exchange chromatog. with an eluent contg. a copper salt to directly sep. amino acids without derivatization.
~4 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
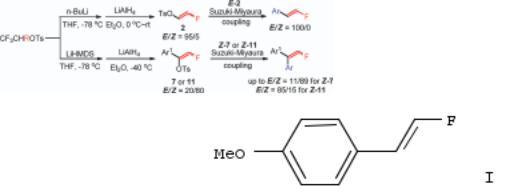
103. Monofluorovinyl Tosylate: A Useful Building Block for the Synthesis of Terminal Vinyl Monofluorides via SuzukiMiyaura Coupling
By Zhang, He; Zhou, Chang-Bing; Chen, Qing-Yun; Xiao, Ji-Chang; Hong, Ran
From Organic Letters (2011), 13(4), 560-563. Language: English, Database: CAPLUS, DOI:10.1021/ol102645g
Monofluorovinyl tosylate was developed as a practical vinyl fluoride building block to couple with a variety of arylboronic acids in the presence of a palladium catalyst. The high stereoselectivity of 2-aryl-1-fluoroethene derivs., e.g., I was achieved. This approach is also applicable to the synthesis of 2,2-diaryl-1-fluoroethenes in good yields.
~12 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
104. Novel cyclopentane derivatives as cysteine protease cathepsin inhibitors and their preparation and use in the treatment of diseases
By Banner, David; Ceccarelli, Simona M.; Grether, Uwe; Haap, Wolfgang; Hilpert, Hans; Kuehne, Holger; Mauser, Harald; Plancher, Jean-Marc; Sanchez, Ruben Alvarez
From PCT Int. Appl. (2010), WO 2010142650 A1 20101216, Language: English, Database: CAPLUS
The invention relates to compds. of formula I which are preferential inhibitors of the cysteine protease cathepsin (Cat), in particular of the cysteine protease cathepsin S or L, making them useful as medicaments, particularly in the treatment of diabetes, atherosclerosis, abdominal aortic aneurysm, peripheral arterial disease or diabetic nephropathy. Compds. of formula I wherein A1 is O, CO, CH2O, CH2, CONH and derivs. and absent; R1 is H, alkyl, haloalkyl, cycloalkyl, Ph, etc.; R2 is H; R3 and R4 are independently H, alkyl and phenyl; R3R4 taken together to form cycloalkyl, alkylpiperidinyl, and alkoxycarbonylpiperidinyl; R2R3 taken together to form pyrrolidinyl; R5 is alkyl, cycloalkyl, cycloalkylalkyl, Ph, etc.; and pharmaceutically acceptable salts thereof, are claimed. Example compd. II was prepd. by a multistep procedure (procedure given). All the invention compds. were evaluated Cat inhibitory activity. From the assay, it was detd. that compd. II exhibited and IC50 value of 0.0006 μM.
