
Reference_01_08_2014_165529
.pdf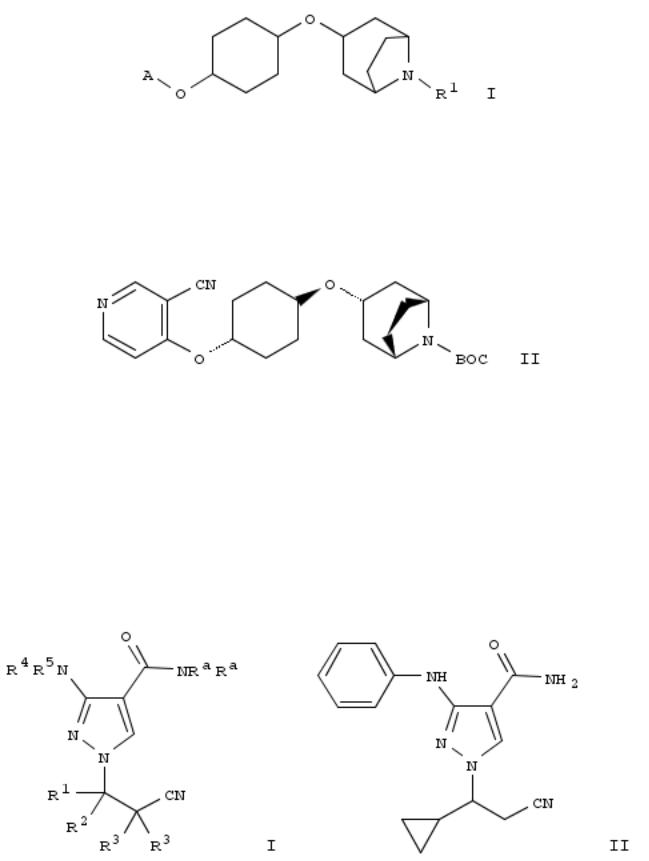
SciFinder® |
Page 11 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
13. Cyanoethylpyrazolecarboxamides as janus kinase inhibitors and their preparation
By Brubaker, Jason; Close, Joshua T.; Jung, Joon; Martinez, Michelle; White, Catherine; Wilson, Kevin; Young, Jonathan R.; Zhang, Hongjun
From PCT Int. Appl. (2013), WO 2013043964 A1 20130328, Language: English, Database: CAPLUS
The invention provides compds. of formula I which are JAK inhibitors, and as such are useful for the treatment of JAKmediated diseases such as rheumatoid arthritis, asthma, COPD and cancer. Compds. of formula I wherein Ra and R4 are independently H and C1-4 alkyl; R1, R2 and R3 are independently H, halo, C1-10 alkyl, C2-10 alkenyl, etc.; R5 is (un)substituted aryl and (un)substituted heteroaryl; and pharmaceutically acceptable salts and stereoisomers thereof, are claimed. Example compd. II was prepd. by conjugate addn. of 3-(phenylamino)-1H-pyrazole-4-carboxamide to (2Z)-3- cyclopropylprop-2-enenitrile. The invention compds. were evaluated for their JAK inhibitory activity (data given).
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
SciFinder® |
Page 12 |
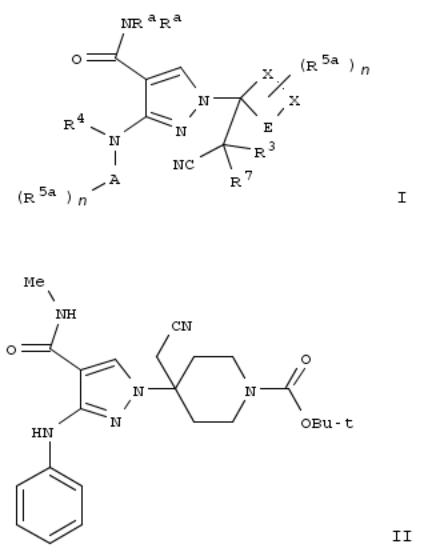
14. Cyanomethylpyrazolecarboxamides as janus kinase inhibitors and their preparation
By Brubaker, Jason; Childers, Matthew Lloyd; Christopher, Matthew; Close, Joshua T.; Katz, Jason David; Jung, Joon; Peterson, Scott; Siliphaivanh, Phieng; Siu, Tony; Smith, Graham Frank; et al
From PCT Int. Appl. (2013), WO 2013043962 A1 20130328, Language: English, Database: CAPLUS
The invention provides compds. of formula I which are JAK inhibitors, and as such are useful for the treatment of JAKmediated diseases such as rheumatoid arthritis, asthma, COPD and cancer. Compds. of formula I wherein Ra and R4 are independently H and C1-4 alkyl; A is aryl and heteroaryl; n is 0, 1, 2, 3, and 4; E is (CH2)0-3; each X is independently C, N, O and S; R3 and R7 are independently H, halo, C1-10 alkyl, C2-10 alkenyl, etc.; R5a is halo, heteroarylsulfonyl, C1-10 alkylsulfonyl, amino, CN, etc.; and pharmaceutically acceptable salts and stereoisomers thereof, are claimed. Example compd. II was prepd. by conjugate addn. of 5-amino-1H-pyrazole-4-carboxylic acid Me ester to tert-Bu 4- (cyanomethylidene)piperidine-1-carboxylate; the resulting tert-Bu 4-[3-amino-4-(methoxycarbonyl)-1H-pyrazol-1-yl]-4- (cyanomethyl)piperidine-1--carboxylate underwent arylation with bromobenzene to give tert-Bu 4-(cyanomethyl)-4-[4- (methoxycarbonyl)-3-(phenylamino)-1H-pyrazol-l-yl]piperidine-1-carboxylate, which underwent hydrolysis to give 1-[1- (tert-butoxycarbonyl)-4-(cyanomethyl)piperidin-4-yl]-3-(phenylamino)-1H-pyrazole-4-carboxylic acid, which underwent amidation with methylamine to give compd. II. The invention compds. were evaluated for their JAK inhibitory activity (data given).
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
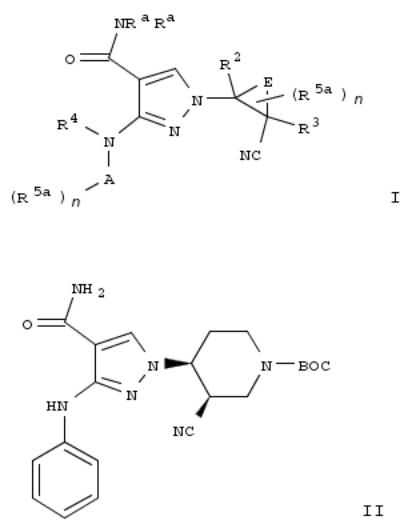
15. Pyrazolecarboxamides as janus kinase inhibitors and their preparation
By Brubaker, Jason; Close, Joshua; Siu, Tony; Smith, Graham Frank; Torres, Luis E.; Woo, Hyun Chong; Young, Jonathan R.; Wei, Zhongyong; Shi, Feng
From PCT Int. Appl. (2013), WO 2013041042 A1 20130328, Language: English, Database: CAPLUS
SciFinder® |
Page 13 |
The invention provides compds. of formula I which are JAK inhibitors, and as such are useful for the treatment of JAKmediated diseases such as rheumatoid arthritis, asthma, COPD and cancer. Compds. of formula I wherein Ra and R4 are independently H and C1-4 alkyl; A is aryl and heteroaryl; n is 0, 1, 2, 3, and 4; E is Xn; n is 2, 3 and 4; X is independently C, N, O and S, provided that at least one X is C; R2 and R3 are independently H, halo, C1-10 alkyl, C2-10 alkenyl, etc.; R5a is H, halo, C1-10 alkylsulfonyl, amino, CN, etc.; and pharmaceutically acceptable salts and stereoisomers thereof, are claimed. Example compd. II was prepd. conjugate addn. of 3-(phenylamino)-1H-pyrazole-4- carboxamide to tert-Bu 5-cyano-3,6-dihydropyridine-1(2H)-carboxylate followed by sepn. of diastereoisomers. The invention compds. were evaluated for their JAK inhibitory activity (data given).
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
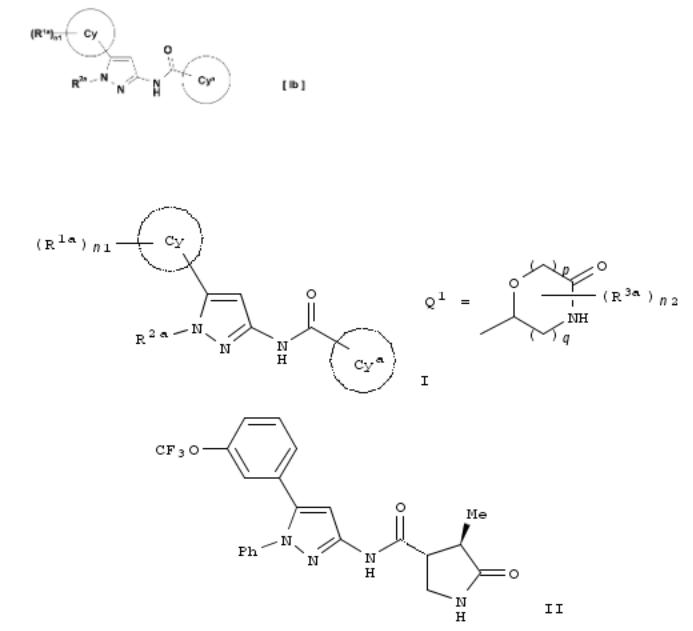
16. Preparation of pyrazole compounds as SGLT1 inhibitors
By Miura, Tomoya; Ogoshi, Yosuke; Ueyama, Kazuhito; Motoda, Dai; Iwayama, Toshihiko; Suzawa, Koichi; Nagamori, Hironobu; Ueno, Hiroshi; Takahashi, Akihiko; Sugimoto, Kazuyuki
From PCT Int. Appl. (2013), WO 2013031922 A1 20130307, Language: Japanese, Database: CAPLUS
SciFinder® |
Page 14 |
Title compds. I [ring Cy = aryl, cycloalkyl or cycloalkenyl; n1 = 0-4; R1a = halo, hydroxy, carboxy, etc.; R2a = alkyl, cycloroalkyl-alkyl, aryl-alkyl, etc.; ring Cya = Q1, etc.; p = 1 or 2; q = 1 or 2; n2 = 0-4; R3a = hydroxy, alkyl or hydroxyalkyl; or pharmaceutically acceptable salts thereof], useful for the treatment of type II diabetes, were prepd. For example, oxidn. of 1-phenyl-4,5-dihydro-1H-pyrazol-3-ylamine, reaction with 2,5-hexanedione, iodination, treatment with hydroxylamine·HCl, Pd(OAc)2-catalyzed coupling reaction with 3- (trifluoromethoxy)phenylboronic acid, and WSC·HCl-mediated acylation with (3R,4R)-4-methyl-5-oxopyrrolidine-3-carboxylic acid afforded compd. II. In SGLT1 inhibition assay, IC50 of II was 0.004 μM. Pharmaceutical compns. comprising II are disclosed.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
17. Preparation of phenylpyrimidinylcarboxylic acid derivatives and analogs for use as kynurenine-3-monooxygenase inhibitors
By Courtney, Stephen Martin; Prime, Michael; Mitchell, William; Brown, Christopher John; De Aguiar Pena, Paula C.; Johnson, Peter; Dominguez, Celia; Toledo-Sherman, Leticia M.; Munoz, Ignacio
From PCT Int. Appl. (2013), WO 2013033085 A1 20130307, Language: English, Database: CAPLUS
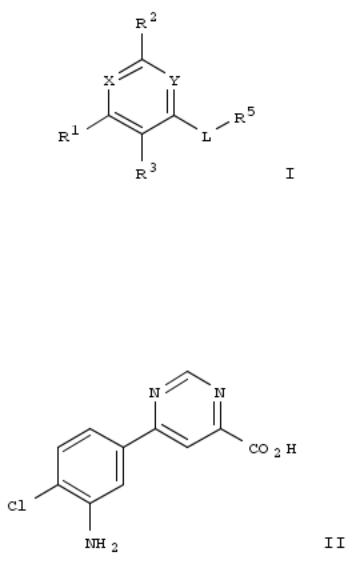
Title compds. I [L = C(O), C(O)O, S(O)2NH, etc.; X and Y independently = N or CH, provided at least one is N; R1 = (un)substituted aryl, monocyclic heteroaryl, 1,3-benzoxazol-5-yl, isoindolin-5-yl, etc.; R2 = H or (un)substituted alkyl; R3 = H, halo, OH, (un)substituted alkyl, etc.; or R1 and R3 taken together to form an (un)substituted bicyclic ring; R5 = H, (un)substituted alkyl, aryl, heteroaryl, etc.; or R3 and R5 taken together to form an (un)substituted ring], and their pharmaceutically acceptable salts, are prepd. and disclosed as kynurenine-3-monooxygenase inhibitors. Thus, e.g., II was prepd. by coupling of 4-chloro-3-nitro-benzene boronic acid and 4,6-dichloropyrimidine followed by carbonylation. Select I were evaluated in inhibition assays, e.g., II demonstrated 101.01% inhibition at 10 μM.
SciFinder® |
Page 15 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
18. Preparation of phenyl-3-azabicyclo[3.1.0]hex-3-yl-methanones as GlyT1 inhibitors
By Giovannini, Riccardo; Bertani, Barbara; Ferrara, Marco; Lingard, Iain; Mazzaferro, Rocco; Rosenbrock, Holger From PCT Int. Appl. (2013), WO 2013017657 A1 20130207, Language: English, Database: CAPLUS
SciFinder® |
Page 16 |
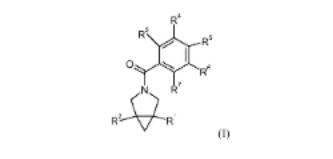
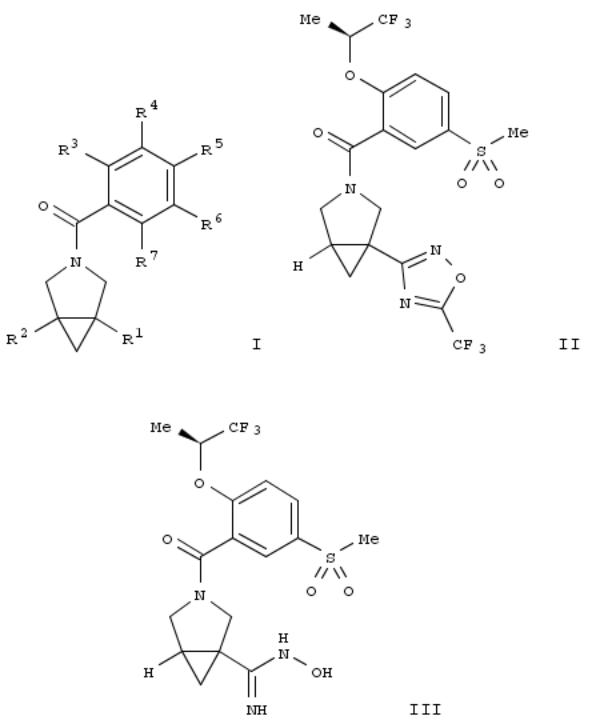
The inventions relates to substituted phenyl-3-aza- bicyclo[3.1.0]hex-3-yl-methanones of formula I and pharmaceutically acceptable salts thereof. The invention further relates to the manuf. of said compds., pharmaceutical compns. comprising a compd. according to formula I, and the use of said compds. for the treatment of various conditions. The compds. of the invention show glycine transporter-1 (GlyT1) inhibiting properties. Compds. of formula I wherein R1 is (un)substituted 5- to 6-membered heteroaryl, (un)substituted 5- to 6-membered heterocyclyl, and (un)substituted 9- to 10membered bicyclic heteroaryl; R2 is H, (un)substituted C1-4 alkyl, (un)substituted C1-4 alkoxy, CN and (un)substituted C3-6 cycloalkyl; R3 is (un)substituted C1-6 alkoxy, (un)substituted C3- 6 cycloalkoxy, morpholino, etc.; R4 is H; R3R4 can ne taken together to form (un)satd. 4- to 6-membered heterocyclyl; R5 is H; R6 is H, C1-4 alkyl-SO2, C3-6 cycloalkyl-SO2 and CN; R7 is H; one of R6R7 or R5R6 may be taken together fo form (un)satd. 5- to 6-membered heterocyclyl; and salts thereof, are claimed. Example compd. II was prepd. by heterocyclization of compd. III with trifluoroacetic anhydride. All the invention compds. were evaluated for their GlyT1 inhibitory activity. From the assay, it was detd. that compd. II exhibited IC50 value of 39 nM.
SciFinder® |
Page 17 |
~1 Citing
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
19. Preparation of substituted (3-phenyl-4,4,4-trifluorobut-1-en-1-yl)benzene compounds useful in pesticidal compositions
By Hunter, James E.; Lo, William C.; Watson, Gerald B.; Patny, Akshay; Gustafson, Gary D.; Pernich, Dan; Brewster, William K.; Camper, Debra L.; Lorsbach, Beth; Loso, Michael R.; et al
From PCT Int. Appl. (2012), WO 2012177813 A1 20121227, Language: English, Database: CAPLUS
SciFinder® |
Page 18 |
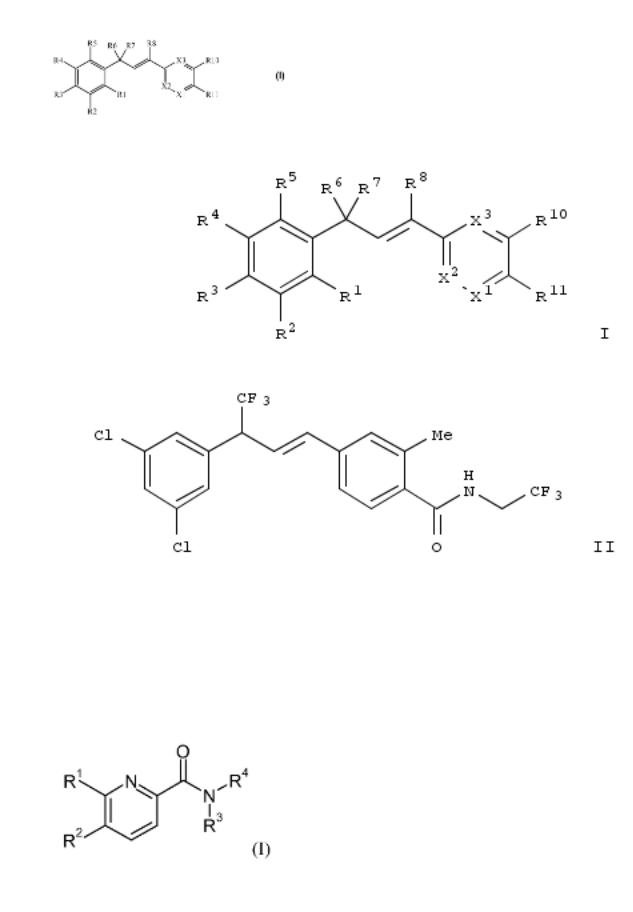
The title compds. I [R1-R5 = H, F, Cl, CN, etc.; R6 = haloalkyl; R7 = H, F, Cl, OH, etc.; R8 = H, alkyl, haloalkyl, etc.; R10 = H, F, Cl, CN, etc.; R11 = (un)substituted alkyl, CO2H, C(O)Oalkyl, etc.; X1 = N, CR12; X2 = N, CR9, CR13; X3 = N, CR9 (wherein R9 = H, F, Cl, alkyl, etc.; R12, R13 = H, F, Cl, CN, etc.)], useful as pesticides, were prepd. Thus, reacting (E)-4-[3-(3,5- dichlorophenyl)-4,4,4-trifluorobut-1-enyl]-2-methylbenzoic acid (prepn. given) with 2,2,2-trifluoroethylamine afforded 50% II. Exemplified compds. I were tested in bioassays on beet armyworm and corn earworm (data given). Compns. comprising I were disclosed.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
20. Preparation of pyridin-2-amides useful as CB2 agonists
By Bissantz, Caterina; Grether, Uwe; Hebeisen, Paul; Kimbara, Atsushi; Liu, Qingping; Nettekoven, Matthias; Prunotto, Marco; Roever, Stephan; Rogers-Evans, Mark; Schulz-Gasch, Tanja; et al
From PCT Int. Appl. (2012), WO 2012168350 A1 20121213, Language: English, Database: CAPLUS
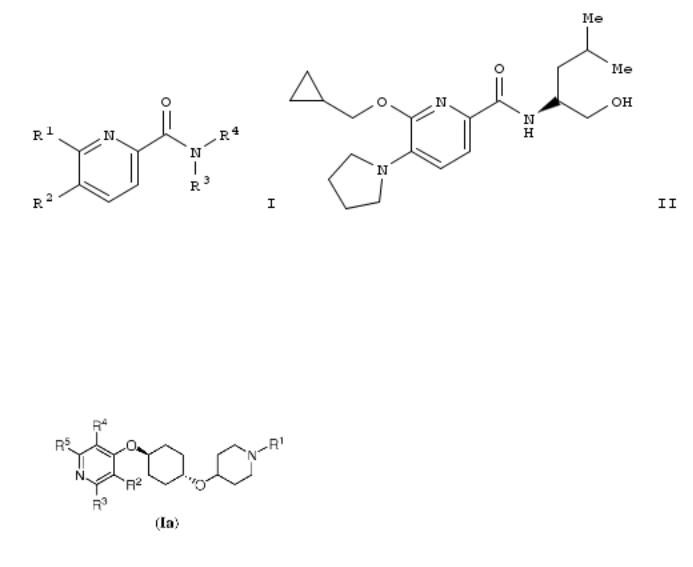
The invention relates to the prepn. of pyridin-2-amides of formula I and pharmaceutically acceptable salts and esters thereof as CB2 agonists. Compd. of formula I wherein R1 is cycloalkyl, haloalkoxy, pyridinylalkoxy, etc.; R2 is H, halo, (halo)alkyl, alkylsulfonyl, etc.; R3 and R4 together with N form piperidinyl, thiomorpholinyl, 1-hydroxyalkylpyrrolidinyl, etc., and pharmaceutically acceptable salts and esters thereof, are claimed. Example compd. II was prepd. by amination of 5- bromo-6-(cyclopropylmethoxy)pyridine-2-carboxylic acid with pyrrolidine; the resulting 6-cyclopropylmethoxy-5-pyrrolidin-1- ylpyridine-2-carboxylic acid underwent amidation with (2S)-2- amino-4-methyl-1-pentanol. All the invention compds. were evaluated for their CB2 agonistic activity. From the assay it was detd. that example compd. II exhibited EC50 values of 0.003 μM and >10 μM towards CB2 and CB1, resp.
SciFinder® |
Page 19 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
21. Preparation of cyclohexyloxy piperidinyl compounds as modulators of the GPR119 receptor and the treatment of disorders related thereto
By Jones, Robert M.; Buzard, Daniel J.; Han, Sangdon; Kim, Sun Hee; Lehmann, Juerg
From PCT Int. Appl. (2012), WO 2012170702 A1 20121213, Language: English, Database: CAPLUS
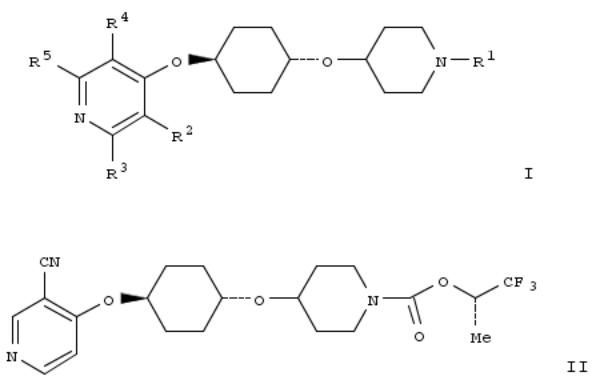
The present invention relates to compds. of Formula I (wherein R1 is C(O)OR6, C(O)R6, C(S)-OR6, CH2R6, or (un)substituted heteroaryl; R6 is C1-6 alkyl, etc.; R2, R3, R4, and R5 are independently H, C1-C14-alkoxy, (un)substituted C1-C4-alkyl, etc.; or R2 and R3 together form part of an (un)substituted heteroaryl),and pharmaceutically acceptable salts, solvates, hydrates, and N-oxides thereof. I are useful as single pharmaceutical agents or in combination with one or more addnl. pharmaceutical agents, such as, an inhibitor of DPP-IV, a biguanide, an alpha-glucosidase inhibitor, an insulin analog, a sulfonylurea, an SGLT2 inhibitor, a meglitinide, a thiazolidinedione, or an anti-diabetic peptide analog, in the treatment of, for example, a disorder selected from: a GPR119- receptor-related disorder; a condition ameliorated by increasing secretion of an incretin; a condition ameliorated by increasing a blood incretin level; a condition characterized by low bone mass; a neurol. disorder; a metabolic-related disorder; type 2 diabetes; obesity; and complications related thereto. Synthetic procedures for prepg. I are exemplified. Example compd. II was prepd. in a 3-step synthesis that culminated in reaction of
(S)-1,1,1-trifluoropropan-2-ol and 4-[(1r,4r)-4-(piperidin-4- yloxy)cyclohexyloxy]nicotinonitrile. In various assays, II was found to be a GPR119 agonist and lowered blood glucose in male 129SVE mice after challenge with glucose.
SciFinder® |
Page 20 |
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
22. Helical twisting power of three-ring chiral molecules and polymerization in cholesteric electrolyte solutions
By Hayashi, Hitoshi; Wang, Aohan; Kawabata, Kohsuke; Goto, Hiromasa
From Materials Chemistry and Physics (2013), 137(3), 816-824. Language: English, Database: CAPLUS, DOI:10.1016/j.matchemphys.2012.10.017
A series of chiral three-ring type compds. with rigid shape was employed as chiral inducers for induction of chiral cholesteric liq. crystal (cholesteric LC) from achiral nematic LC. Helical twisting power of the chiral compds. was estd. with the Cano wedge method. Cholesteric LC electrolyte soln. was prepd. by adding the chiral compds. Subsequently, polymn. in the cholesteric LC was carried out to produce chiroptically active polymer films. This method is different from conventional methods for synthesizing chiral polymers because neither chiral monomers nor asym. catalysts are employed. Surface structure and optical properties of the polymer thus prepd. were examd.
~0 Citings
Copyright © 2014 American Chemical Society (ACS). All Rights Reserved.
23. Preparation of novel spiropiperidine derivatives promoting insulin secretion
By Morimoto, Toshiharu; Koshizawa, Tomoaki; Watanabe, Gen; Fukuda, Tomoaki; Ohgiya, Tadaaki; Yamasaki, Nao; Inoue, Noriyuki; Araki, Takaaki; Tsukagoshi, Hitomi; Hagita, Sumihiko
From PCT Int. Appl. (2012), WO 2012161119 A1 20121129, Language: Japanese, Database: CAPLUS
