
Statistical physics (2005)
.pdfChapter 4
The Ideal Gas
4.1Introduction
After having stated the general methods in Statistical Physics and bridged its concepts with your previous background in Thermodynamics, we are now going to apply our know-how to the molecules of a gas, with no mutual interaction. The properties of such a gas, called “ideal gas” (“perfect gas” in French) are discussed in Thermodynamics courses. Here the contribution of Statistical Physics will be evidenced : starting from a microscopic description in Classical Mechanics, it allows to explain the observed macroscopic properties.
A few notions of kinetic theory of gases will first be introduced in §4.2 and §4.3 we will discuss to what extent classical Statistical Physics, as used up to this point in this book, can apply to free particles. This chapter is “doubly classical” since, like in the three preceding chapters, Classical Statistics is used, but now for particles with an evolution described by Classical Mechanics : both the states themselves and their occurrence are classical. The canonical ensemble is chosen in §4.4 to solve the statistical properties of the ideal gas and physical consequences are deduced; in the Appendices, all that we learnt in this chapter and the preceding ones is applied in a practical problem : the interpretation of chemical reactions in gas phase is done in Thermodynamics (Appendix 4.1) and in Statistical Physics (Appendix 4.2).
79
80 |
Chapter 4. The Ideal Gas |
4.2Kinetic Approach
This section, which has no relation with Quantum Mechanics or Statistical Physics in equilibrium, will allow us to obtain insight into the simplest physical parameters describing the motion of molecules in a gas, and to introduce some orders of magnitude. If necessary, we will refer to the Thermodynamics courses on the ideal gas.
4.2.1Scattering Cross Section, Mean Free Path
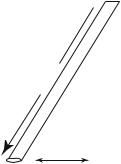
Consider a can filled with a gas, in the standard conditions of temperature and pressure (T = 273 K, P = 1 atmosphere). Each molecule has a radius r, defined by the average extension of the valence orbitals, i.e., a fraction of a nanometer. If in its motion a molecule comes too close to another one, there will be a collision. One defines the scattering cross section which is the circle of radius r around a molecule, inside which no other molecule can penetrate.

 r
r
= |
ντ |
|
|
l |
|
d
Fig. 4.1: Definitions of the scattering cross section and of the mean free path.
The mean distance between two collisions l, or mean free path, is deduced from the mean volume per particle Ω/N and the scattering cross section by
l(πr2) = |
Ω |
= d3 |
(4.1) |
|
N |
||||
|
|
|
where d is the average distance between particles (Fig. 4.1). From l, one deduces the mean time τ between two collisions, or collision time, by writing
l = ντ |
(4.2) |
|||
where ν is the mean velocity at temperature T , of the order of |
|
|
|
|
kB T /m (see |
||||
the justification in §4.4.2). |
||||
Kinetic Approach |
81 |
For nitrogen molecules with r = 0.42 nm, at 273 K and under a pressure of one atmosphere one finds l ≈ 70 nm, ν ≈ 300 m.sec−1, τ ≈ 2 × 10−10 sec. The length l is to be compared with the average distance between particles d = 3.3 nm. These values show that in the gas collisions are very frequent at the time scale of a measure, which ensures that equilibrium is rapidly reached after a perturbation of the gas; at the molecular scale distances covered between collisions are very large (of the order of a hundred times the size of a molecule), thus the motion of each molecule mostly occurs as if it was alone, that is, free.
4.2.2Kinetic Calculation of the Pressure
Let us now recall the calculation, from kinetic arguments, of the pressure of a gas, i.e., of the force exerted on a wall of unit surface by the molecules. The aim is to evaluate the total momentum transferred to this wall by the molecules impinging on it during the time interval ∆t : indeed in this chapter Classical Mechanics does apply, so that the total force on the wall is given by the Fundamental Equation of Dynamics :
|
∆pi |
|
Ftotal = |
∆t |
(4.3) |
i
Here the momentum brought by particle i is ∆pi. The resulting force is normal since the wall is at rest, it is related to the pressure P and to the considered wall surface S through
|
(4.4) |
|Ftotal| = P S |
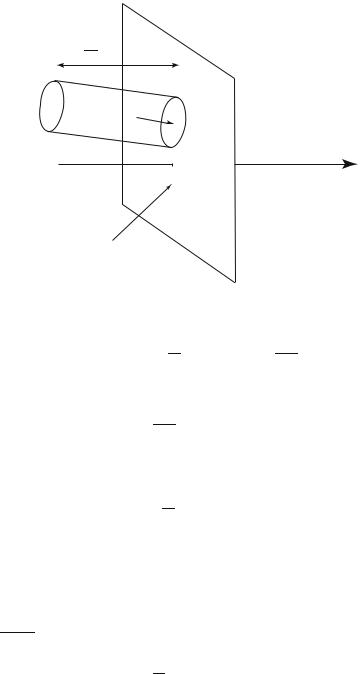
One will now evaluate the total momentum transferred to the surface, which is the di erence between the total momentum of the particles which reach the wall and that of the particles which leave it (Fig. 4.2).
The incident particles, with pz > 0, bring a total momentum |
pif (r, pi, t) |
to the wall, chosen as xOy plane. |
i |
|
The probability density in equilibrium f (r, pi, t) is assumed to be stationary, homogeneous, and isotropic in space (hypotheses stated by James Clerk Maxwell in 1859). It thus reduces to an even function f (p) versus the three components of the momentum and satisfies to
f (p)d3p = 1 |
(4.5) |
Consider the particles, of momentum equal to p to d3p, incident on the surface S during the time interval ∆t. They are inside an oblique cylinder along the
82 |
Chapter 4. The Ideal Gas |
pmz ∆t
S p
O |
z |
p
Fig. 4.2 : Collisions of molecules on a wall.
direction of p, of basis S, height pmz ∆t and volume Smpz ∆t (the direction z is
chosen along the normal to the surface). For a particles density ρ = N/Ω, the number of incident particles is
Spz |
∆t ρf (p)d3p |
(4.6) |
m |
Each of them transfers its momentum p to the wall. The total momentum brought by the incident particles is thus equal to
inc |
i |
|
m |
pz >0 |
z |
|
|
|
∆p |
|
= |
S |
∆tρ |
|
p |
pf (p)d3p |
(4.7) |
|
|
|
||||||
This sum is restricted to the particles traveling toward the surface, of momentum pz > 0.
A similar calculation leads to the total momentum of the particles which leave the surface, or in other words “were reflected,” by the wall. The volume
is S (−pz ) ∆t, the total momentum taken from the wall is equal to m
refl |
i |
|
m |
pz <0 |
− z |
|
|
|
∆p |
|
= |
S |
∆tρ |
( |
p |
)pf (p)d3p |
(4.8) |
|
|
|||||||
The net total momentum transferred to the wall is obtained by integrating on

Classical or Quantum Statistics ? |
83 |
all values of momentum pz , i.e.,
inc |
i − refl |
i |
|
m |
|
z |
|
|
|
∆p |
|
∆p |
= |
S |
∆tρ |
|
p |
pf (p)d3p |
(4.9) |
Because the probability is an even function, the integrals in pxpz and in pypz vanish, so that the integral reduces to the z component, normal to the surface :
inc ∆pi − rEfl ∆pi |
z |
= 2S ∆tρ |
p2 |
(4.10) |
2m f (p)d3p |
||||
|
|
|
z |
|
It is related to the average value of the contribution to the kinetic energy of the motion along Oz : since the distribution of momenta is taken as isotropic, this is one third of the average value of the total kinetic energy Ec. One can deduce the expression of the normal force on the wall and thus of the pressure :
F |
= 2 |
S |
ρ |
Ec |
(4.11) |
|
total |
|
3 |
|
|||
P = 2ρ |
Ec |
(4.12) |
||||
|
|
|
|
3 |
|
|
As you already know, and will be again shown in this chapter in §4.4.2, the average kinetic energy per particle in a monoatomic ideal gas, with only translational degrees of freedom, is equal to
Ec = ≠ |
p2 |
∑ = |
Æ |
p2 |
+ py2 + p2 |
∏ = |
3 |
|
|
|
|
x |
|
z |
|
kB T |
(4.13) |
||||
2m |
|
2m |
|
2 |
||||||
Consequently, the pressure is related to the particles density and to the temperature through
P = 2ρ |
1 |
· |
3 |
kB T = ρkB T = |
N |
(4.14) |
|
|
|
|
kB T |
||||
3 |
2 |
Ω |
|||||
a relation which is the well-known ideal gas law.
This type of kinetic calculation will be used in chapter 9 on thermal radiation; in the case of nonequilibrium Statistical Physics, it also allows one to estimate the order of magnitude of transport coe cients (for example, thermal conductivity, viscosity).
4.3Classical or Quantum Statistics ?
We now consider the range of validity of Classical Statistical Physics for studying the properties of a gas of free molecules.
84 |
Chapter 4. The Ideal Gas |
It is well known that the correct treatment of the particles motion must be done in Quantum Mechanics, so that the motion of a particle should not be described in terms of a trajectory but rather of a probability of presence deduced from a wave function. Moreover, the specific question raised by the Quantum Statistics, as will be seen in Chapter 5, is that of the indistinguishability of the particles.
But you also learnt that Classical Mechanics can however be used when the size of the considered system, here the average distance between two particles, is much larger than the characteristic dimension of the wave function, here its wavelength (in the opposite situation, the description in Quantum Mechanics is mandatory).
Let us first evaluate the characteristic dimension, at a given temperature T , of the wavelength of the molecules’ wave function. The momenta distribution is a function of T : it was just recalled that the mean kinetic energy per particle
of an ideal gas is equal to 32 kB T in thermal equilibrium at temperature T , which gives
» |
p2 |
= |
3mkB T |
(4.15) |
||
Using the Louis de Broglie relation (1926), |
|
|||||
|
|
λ = |
h |
|
(4.16) |
|
|
|
p |
||||
|
|
|
|
|||
to the root-mean-square value (4.15) of the momentum, one associates the wavelength λ of a free particle wave function, where h is the Planck constant. In order to simplify the forthcoming expressions of §4.4, the thermal de Broglie wavelength is defined here by
√ |
|
|
|
|
|
|
|
|
|
|
λth = √ |
|
3 |
|
|
|
h |
= |
√ |
h |
(4.17) |
|
|
|
|
|
|
|
||||
2π |
|
p2 |
2πmkB T |
|||||||
To know which of the Classical or Quantum Statistics applies, one thus compares λth to the averagedistance d between particles, of the order of (Ω/N )1/3.
Ω
If λth d, λ3th N , the classical statistics is applicable. Then
|
|
h |
|
|
|
|
Å |
Ω |
ã |
1/3 |
|
||
|
|
|
|
|
|
|
(4.18) |
||||||
|
|
|
|
|
|
|
|
||||||
|
√ |
2πmkB |
1/3 |
N |
|
||||||||
|
|
|
|
T |
|
|
|
|
|
||||
h Å |
Ω |
ã |
|
|
|
|
|
|
|
|
|||
|
2πmkB T |
(4.19) |
|||||||||||
|
|
||||||||||||
N |
|
||||||||||||
This is a high temperature or low density regime.
Classical Statistics Treatment of the Ideal Gas |
85 |
On the other hand, for λth ≥ d, the treatment of the present chapter is no longer valid and a quantum analysis is necessary (see Chapter 5 and following ones).
Take the example of a nitrogen gas in standard conditions of temperature and pressure : Ω = 22.4 liters for N = 6.02 × 1023 molecules and T = 273 K. The quantity to be compared to h = 6.63 × 10−34 J.sec is equal here to 1 × 10−31. Indeed the regime is classical. On the other hand, when at constant pressure the temperature is lowered to a few kelvins, the thermal L. de Broglie wavelength and the average distance between particles become comparable for gases of low atomic mass like helium (under the condition that they are not solid under these conditions!). In fact the right member of (4.19) is also written (kB T /P )1/3(2πmkB T )1/2 and thus varies like m1/2T 5/6. Classical Statistics is then no longer valid (see chapter 9).
In the next section we will study the ideal gas by the classical “MaxwellBoltzmann” statistics and thus assume that the condition λth d is fulfilled. Consequently, a classical description of the motion and of the phase space can be used (see §(1.1)) : until the next chapter we forget Quantum Mechanics...
4.4Classical Statistics Treatment of the Ideal Gas in the Canonical Ensemble
As explained in chapter 2, the statistical treatment of a macroscopic system will give the same physical results, whether performed in the microcanonical, canonical or grand canonical ensemble. In the ideal gas case, the microcanonical ensemble leads to somewhat more complicated calculations. The treatment could be done in the grand canonical ensemble; here we will choose the canonical ensemble. We follow the general method (see chapter 3) consisting in first calculating the canonical partition function Zc (§4.4.1), then deducing the average energy (§4.4.2) and the free energy F (§4.4.3) the partial derivatives of which provide the thermodynamical parameters pressure, entropy and chemical potential.
4.4.1Calculation of the Canonical Partition Function
We are concerned here by N monoatomic molecules, confined into a volume Ω, with energies reduced to their translational kinetic energies within this volume. Consequently, the probability of occurrence of the state in which the molecule i is within the volume d3ri around ri, with the momentum pi to
86 Chapter 4. The Ideal Gas
d3pi, is given by |
|
2m + V (ri) |
|
|
|
|
|
|||
|
Zc exp −β |
|
|
h3N |
|
(4.20) |
||||
1 |
i |
Å |
p2 |
ã |
|
C d3r d3p |
i |
|
||
|
|
i |
|
i |
|
|||||
|
|
|
|
|
|
N i |
|
|||
To express the confinement, the potential energy is taken as zero inside the volume Ω, infinite outside it. The canonical partition function for N particles, which normalizes the probabilities to unity, is calculated using the measure of the 6N -dimensional phase space introduced in §1.2.2 and expressed in the above formula. (Remember that CN is a constant which only depends on the number of particles.)
Zc = exp −β |
|
|
2m + V (ri) |
|
|
h3N |
|
(4.21) |
|
|
|
Å |
pi2 |
ã |
i |
CN d3rid3 |
pi |
|
|
i |
|
|
|
|
|
||||
As already seen in §2.4.5 of chapter 2, since the total energy consists in a sum of similar one-particle terms, the partition function is a product of terms :
|
|
Zc = CN (z1)N |
|
|
|
|
(4.22) |
|||||
with |
|
|
|
|
|
|
|
|
|
|
|
|
z1 |
= |
exp |
β |
p12 |
|
|
+ V (r1) |
d3r1d3p1 |
(4.23) |
|||
|
|
|
3p h3 |
|||||||||
|
|
ï− |
Å |
2m |
2 |
ãò |
|
|
||||
|
= |
e−βV (r1)d3r1 |
|
e−βp1 |
/2m |
d 1 |
|
(4.24) |
||||
|
h3 |
|||||||||||
(Do not forget the CN factor of the phase space measure!)
If the potential only expresses that the molecule remains within the volume Ω, the integral in r1 is equal to Ω (the exponential is equal to one in the allowed volume, zero outside it).
Note : If the potential is position-dependent, as in the case of a gas in equilibrium in the gravity field, the probability of presence and the density both vary in exp(−βV (r)) and the integral in r1 is di erent from Ω (it is proportional to the characteristic extension of the potential energy).
From now we assume that the gas is confined, with a uniform probability of presence within the allowed volume.
The integral in momentum is reduced into the product of three Gaussian integrals, each one concerning a single coordinate :
|
|
p12 |
|
d3p1 |
|
+∞ |
|
p12x |
|
dp1x |
ô |
3 |
exp −β |
|
= |
exp −β |
|
(4.25) |
|||||||
2m |
ã |
h3 |
ñ −∞ |
2m |
ã |
h |
||||||
|
Å |
|
|
|
|
Å |
|
|
|
|

Classical Statistics Treatment of the Ideal Gas |
87 |
Homogeneityconsiderations predict a result with a dimension (momentum/h)3. |
||||||
Now the characteristic momentum in the exponential is |
|
= |
√ |
|
. The |
|
m |
||||||
mkB T |
||||||
|
β |
|
|
|
||
mathematical appendix at the end of this book gives the value of the dimen- |
||||||
sionless Gaussian integral. Finally |
|
|
» |
|||
|
Ω |
|
Ω |
|
||
z1 = |
|
|
(2πmkB T )3/2 |
= |
|
(4.26) |
h |
3 |
3 |
||||
|
|
|
|
λ |
|
|
|
|
|
|
|
th |
|
where the thermal de Broglie wavelength, defined in §4.3, is expressed. One deduces the N -particle canonical partition function
|
Ω |
N |
|
Ω |
N |
|
Å |
ã |
Å |
ã |
|||
|
|
|||||
h3 |
λth3 |
|||||
Zc = CN |
|
(2πmkB T )3N/2 = CN |
|
|
(4.27) |
that we will use to calculate the free energy F . But before that we will calculate the average value of the N -molecule kinetic energy, using Maxwell’s original method.
4.4.2Average Energy; Equipartition Theorem
In the absence of potential energy other than the confinement one, the internal energy in the sense of Thermodynamics reduces to this average kinetic energy. By definition :
Ec = |
i |
2m exp ñ−β( j |
2m + V (rj ))ô |
i |
N h3N |
(4.28) |
|||||
|
|
|
pi2 |
|
|
pj 2 |
|
C |
d3rid3pi |
|
|
|
|
ï−β( i |
2 |
|
3 |
3 |
|
||||
|
exp |
2m |
+ V (ri))ò i |
N h3N |
|
||||||
|
|
|
|
pi |
|
C |
d rid pi |
|
|||
The integrals of this complicated expression factorize both in the numerator and in the denominator, the particle i playing a specific role :
Ec =
×
|
|
|
|
d3rid3pi pi2 |
ñ−β( |
pi2 |
|
|||||||||||
|
|
|
|
|
|
|
|
|
exp |
|
+ V (ri))ô |
(4.29) |
||||||
i |
|
h3 |
2m |
2m |
||||||||||||||
j=i |
|
|
CN d3rj d3pj |
|
|
− |
|
2 2 |
|
|
|
|
|
|||||
CN d3rj d3pj |
|
exp |
|
pj |
+ V (rj )) |
|
|
|||||||||||
|
|
|
|
h3 |
|
β( m |
|
|||||||||||
j |
|
|
|
|
|
|
exp î−β( |
|
+ V (rj ))ó |
|
||||||||
|
|
h3 |
|
2m |
|
|||||||||||||
As for particle i, one separates the terms corresponding to the three degrees of freedom xi, yi and zi. A sum of 3N analogous terms appears :
|
|
= 3N |
|
+∞ px2 |
|
|
|
px2 |
|
|
|
|||
Ec |
|
|
|
|
|
|
|
|
|
|
(4.30) |
|||
|
+ |
|
|
|
px2 |
|
|
|||||||
|
|
−∞∞ exp |
Ä−β |
2m |
ä dpx |
|||||||||
|
|
|
|
−∞ 2m exp |
−β 2m |
|
dpx |
|||||||
88 Chapter 4. The Ideal Gas
For each of these terms, an integration by parts allows one to transform the
numerator term into the denominator one to the factor |
1 |
= |
kB T |
, for |
|||||||||||||
|
2β |
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
||
+∞ px2 |
|
px2 |
1 |
|
|
+∞ |
|
px2 |
|
|
|
||||||
−∞ |
|
exp |
Å−β |
|
ã dpx = |
|
|
−∞ exp |
Å−β |
|
ã dpx |
(4.31) |
|||||
2m |
2m |
2β |
2m |
||||||||||||||
Consequently, |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
U = |
3N |
= |
3 |
N kB T |
|
|
|
|
|
(4.32) |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
2β |
2 |
|
|
|
|
|
|||||||
In fact this calculation is valid in the Maxwell-Boltzmann Classical Statistics for the average value of any energy term expressed as a quadratic degree of
freedom, for example an elastic potential energy of the type 12 kix2i , for which
ki > 0. Indeed one will always perform an integration by parts of a seconddegree term in energy in the numerator to express the corresponding factor of the denominator partition function, which will provide the factor kB T /2. But this property is not valid for a polynomial of degree di erent than two, for example for an anharmonic term in x3i .
This result constitutes the “theorem of energy equipartition”, established by James C. Maxwell as early as 1860 :
For a system in canonical equilibrium at temperature T , the average value of the energy per particle and per quadratic degree of
freedom is equal to 12 kB T .
We have already used this property several times, in particular in §4.3 to find the root-mean-square momentum of the ideal gas at temperature T . It is also useful to determine the thermal fluctuations of an oscillation, providing
a potential energy in 12 kx2 for a spring, in 12 Cϑ2 for a torsion pendulum,
and more generally to calculate the average value at temperature T of a hamiltonian of the form
h = a x2 |
+ |
b |
p2 |
(4.33) |
i i |
|
j |
j |
|
ij
where ai is independent of xi, bj does not depend of pj , and all the coe - cients ai and bj are positive (which allows to use this property for an energy development around a stable equilibrium state).
A case of application of the energy equipartition theorem is the specific heat at constant volume associated to U . We recall its expression
Å ∂U ã
Cv = (4.34)
∂T Ω,N
