
- •About the Authors
- •Preface
- •Contents
- •Basics
- •Chemistry
- •Physical Chemistry
- •Biomolecules
- •Carbohydrates
- •Lipids
- •Amino Acids
- •Peptides and Proteins
- •Nucleotides and Nucleic Acids
- •Metabolism
- •Enzymes
- •Metabolic Regulation
- •Energy Metabolism
- •Carbohydrate Metabolism
- •Lipid Metabolism
- •Protein Metabolism
- •Nucleotide Metabolism
- •Porphyrin Metabolism
- •Organelles
- •Basics
- •Cytoskeleton
- •Nucleus
- •Mitochondria
- •Biological Membranes
- •Endoplasmic Reticulum and Golgi Apparatus
- •Lysosomes
- •Molecular Genetics
- •Genetic engineering
- •Tissues and organs
- •Digestion
- •Blood
- •Immune system
- •Liver
- •Kidney
- •Muscle
- •Connective tissue
- •Brain and Sensory Organs
- •Nutrition
- •Nutrients
- •Vitamins
- •Hormones
- •Hormonal system
- •Lipophilic hormones
- •Hydrophilic hormones
- •Other signaling substances
- •Growth and development
- •Cell proliferation
- •Viruses
- •Metabolic charts
- •Annotated enzyme list
- •Abbreviations
- •Quantities and units
- •Further reading
- •Source credits
- •Index
- •Introduction
- •Basics
- •Biomolecules
- •Carbohydrates
- •Lipids
- •Metabolism
- •Organelles
- •Cytoskeleton
- •Nucleus
- •Lysosomes
- •Molecular Genetics
- •Genetic engineering
- •Tissues and organs
- •Digestion
- •Immune system
- •Nutrition
- •Vitamins
- •Hormones
- •Lipophilic hormones
- •Hydrophilic hormones
- •Growth and development
- •Viruses
- •Metabolic charts
- •Annotated enzyme list
- •Abbreviations
- •Quantities and units
- •Further reading
- •Source credits
- •Index

374 Hormones
Lipophilic hormones
Classifying hormones into hydrophilic and lipophilic molecules indicates the chemical properties of the two groups of hormones and also reflects differences in their mode of action (see p. 120).
A. Lipophilic hormones
Lipophilic hormones, which include steroid hormones, iodothyronines, and retinoic acid, are relatively small molecules (300–800 Da) that are poorly soluble in aqueous media. With the exception of the iodothyronines, they are not stored by hormone-forming cells, but are released immediately after being synthesized. During transport in the blood, they are bound to specific carriers. Via intracellular receptors, they mainly act on transcription (see p. 358). Other effects of steroid hor- mones—e.g., on the immune system—are not based on transcriptional control. Their details have not yet been explained.
Steroid hormones
The most important steroid hormones in vertebrates are listed on p.57. Calcitriol (vitamin D hormone) is also included in this group, although it has a modified steroid structure. The most important steroid hormone in invertebrates is ecdysone.
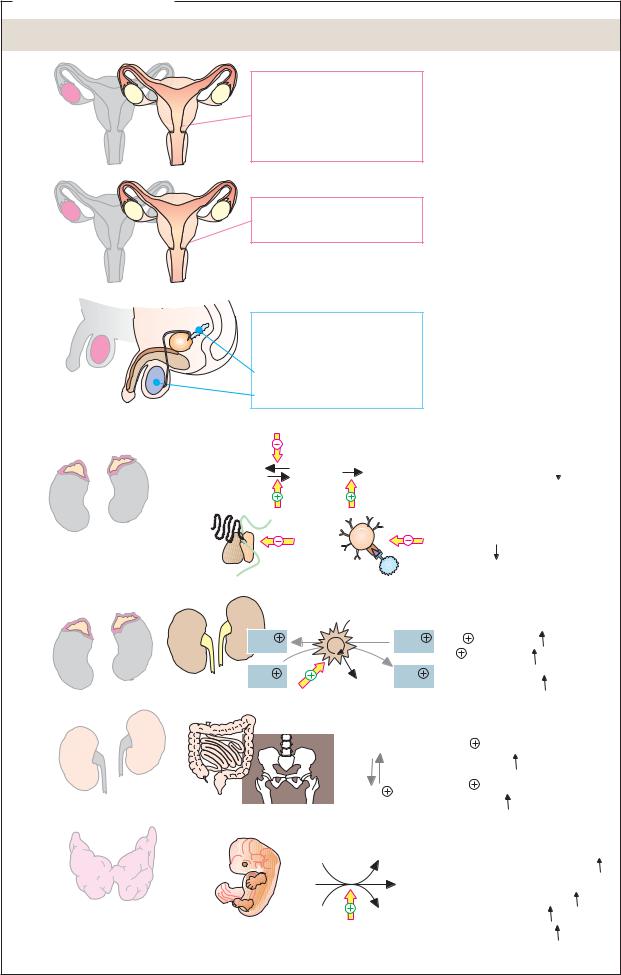
Progesterone is a female sexual steroid belonging to the progestin (gestagen) family. It is synthesized in the corpus luteum of the ovaries. The blood level of progesterone varies with the menstrual cycle. The hormone prepares the uterus for a possible pregnancy. Following fertilization, the placenta also starts to synthesize progesterone in order to maintain the pregnant state. The development of the mammary glands is also stimulated by progesterone.
Estradiol is the most important of the estrogens. Like progesterone, it is synthesized by the ovaries and, during pregnancy, by the placenta as well. Estradiol controls the menstrual cycle. It promotes proliferation of the uterine mucosa, and is also responsible for the development of the female secondary sexual characteristics (breast, fat distribution, etc.).
Testosterone is the most important of the male sexual steroids (androgens). It is synthesized in the Leydig intersitial cells of the testes, and controls the development and functioning of the male gonads. It also determines secondary sexual characteristics in men (muscles, hair, etc.).
Cortisol, the most important glucocorticoid, is synthesized by the adrenal cortex. It is involved in regulating protein and carbohydrate metabolism by promoting protein degradation and the conversion of amino acids into glucose. As a result, the blood glucose level rises (see p. 152). Synthetic glucocorticoids (e.g., dexamethasone) are used in drugs due to their anti-inflammatory and immunosuppressant effects.
Aldosterone, a mineralocorticoid, is also synthesized in the adrenal gland. In the kidneys, it promotes Na+ resorption by inducing Na+/K+ ATPase and Na+ channels (see p. 328). At the same time, it leads to increased K+ excretion. In this way, aldosterone indirectly increases blood pressure.
Calcitriol is a derivative of vitamin D (see p. 364). On exposure to ultraviolet light, a precursor of the hormone can also arise in the skin. Calcitriol itself is synthesized in the kidneys (see p. 330). Calcitriol promotes the resorption of calcium in the intestine and increases the Ca2+ level in the blood.
Iodothyronines
The thyroid hormone thyroxine (tetraiodothyronine, T4) and its active form triiodothyronine (T3) are derived from the amino acid tyrosine. The iodine atoms at positions 3 and 5 of the two phenol rings are characteristic of them. Post-translational synthesis of thyroxine takes place in the thyroid gland from tyrosine residues of the protein thyroglobulin, from which it is proteolytically cleaved before being released. Iodothyronines are the only organic molecules in the animal organism that contain iodine. They increase the basal metabolic rate, partly by regulating mitochondrial ATP synthesis. In addition, they promote embryonic development.
Lipophilic hormones |
375 |
A. Lipophilic hormones
Hormone |
Site of formation |
Sites of action |
|
|
Prepares uterus |
|
|
for pregnancy |
Ovaries |
|
Promotes implantation |
|
|
of fertilized egg |
Progesterone |
Uterus |
|
|
|
Stimulates proliferation |
|
|
of endometrium |
Ovaries |
|
|
Estradiol |
Uterus and other organs |
|
Testes |
|
Causes: |
|
|
Sexual differentiation |
|
|
to male phenotype |
|
|
Formation of ejaculate |
Testosterone |
|
Spermatogenesis |
Proteins |
Amino |
Glucose |
|
acids |
|||
|
|
||
Adrenal glands |
|
|
|
(cortex) |
|
|
|
Cortisol |
|
|
Actions
Maintenance of pregnancy 
Development of mammary glands
Menstrual cycle Bone development
Development of secondary female sex characteristics e.g., fat distribution, breasts, body hair
Development of secondary male sex characteristics e.g., skeleton, muscles, body hair 
Protein synthesis 
Proteolysis  Protein synthesis
Protein synthesis 
Gluconeogenese  Blut-Glucose
Blut-Glucose 
Activity of the immune system
|
|
|
ATP |
|
|
Adrenal glands |
3Na |
3Na |
Na |
retention |
|
|
|
K |
excretion |
||
(cortex) |
|
|
|
||
|
2K |
2K |
|
|
|
Aldosterone |
Kidneys |
Blood pressure |
|||
|
|
ADP+Pi |
|
|
|
Kidneys |
|
|
Bones |
Ca2 |
- and phosphate |
|
|
|
resorption |
||
|
Gut |
|
Ca2 |
Ca2 |
metabolism |
Calcitriol |
|
of bones |
|||
|
|
||||
|
Thyroid gland |
O2 |
H2O |
Fetal development, |
|
|
|
||||
|
|
|
|
growth, and maturation |
|
|
|
S |
CO2 |
Basal metabolic rate |
|
|
|
|
|
||
Thyroxine |
Embryo |
ADP+Pi |
ATP, Heat |
Heat generation |
|
Intermediary |
O2 consumption |
||||
|
|
metabolism |
|
|
|

376 Hormones
Metabolism of steroid hormones
A. Biosynthesis of steroid hormones
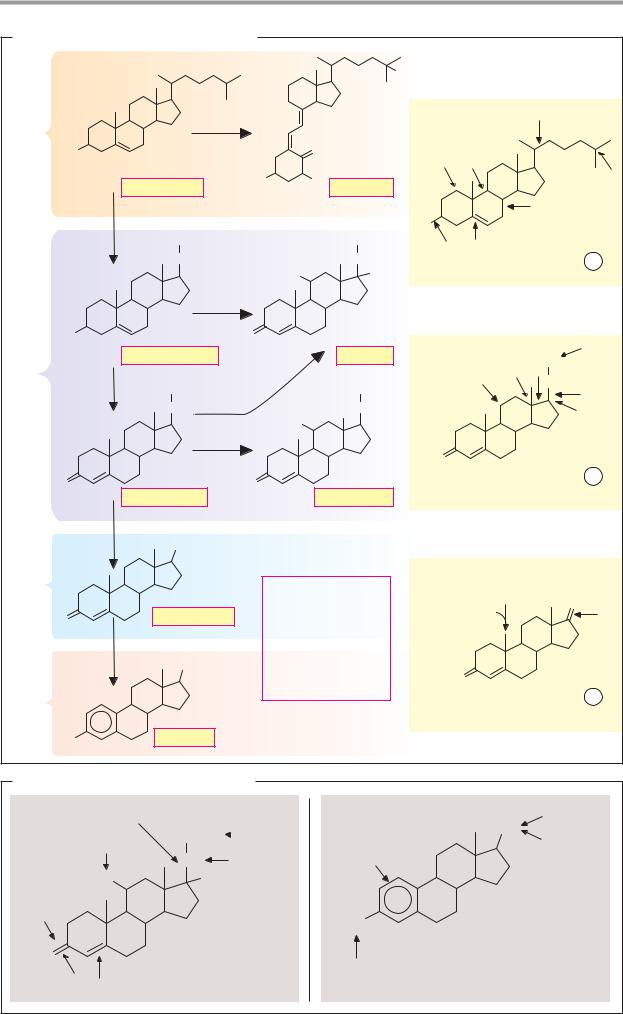
All steroid hormones are synthesized from cholesterol. The gonane core of cholesterol consists of 19 carbon atoms in four rings (A–D). The D ring carries a side chain of eight C atoms (see p. 54).
The cholesterol required for biosynthesis of the steroid hormones is obtained from various sources. It is either taken up as a constituent of LDL lipoproteins (see p. 278) into the hormone-synthesizing glandular cells, or synthesized by glandular cells themselves from acetyl-CoA (see p. 172). Excess cholesterol is stored in the form of fatty acid esters in lipid droplets. Hydrolysis allows rapid mobilization of the cholesterol from this reserve again.
Biosynthetic pathways. Only an overview of the synthesis pathways that lead to the individual hormones is shown here. Further details are given on p.410.
Among the reactions involved, hydroxylations (H) are particularly numerous. These are catalyzed by specific monooxygenases (“hydroxylases”) of the cytochrome P450 family (see p. 318). In addition, there are NADPHdependent and NADP+-dependent hydrogenations (Y) and dehydrogenations (D), as well as cleavage and isomerization reactions (S, I). The estrogens have a special place among the steroid hormones, as they are the only ones that contain an aromatic A ring. When this is formed, catalyzed by aromatase, the angular methyl group (C-19) is lost.
Pregnenolone is an important intermediate in the biosynthesis of most steroid hormones. It is identical to cholesterol with the exception of a shortened and oxidized side chain. Pregnenolone is produced in three steps by hydroxylation and cleavage in the side chain. Subsequent dehydrogenation of the hydroxyl group at C-3 (b) and shifting of the double bond from C-5 to C-4 results in the gestagen progesterone.
With the exception of calcitriol, all steroid hormones are derived from progesterone. Hydroxylations of progesterone at C atoms 17, 21, and 11 lead to the glucocorticoid cortisol. Hydroxylation at C-17 is omitted during synthesis of the mineralocorticoid aldosterone. Instead, the angular methyl group (C-18) is oxidized to the aldehyde group. During syn-
thesis of the androgen testosterone from progesterone, the side chain is completely removed. Aromatization of the A ring, as mentioned above, finally leads to estradiol.
On the way to calcitriol (vitamin D hormone; see p.342), another double bond in the B ring of cholesterol is first introduced. Under the influence of UV light on the skin, the B ring is then photochemically cleaved, and the secosteroid cholecalciferol arises (vitamin D3; see p.364). Two Cyt P450-depen- dent hydroxylations in the liver and kidneys produce the active vitamin D hormone (see p. 330).
B. Inactivation of steroid hormones
The steroid hormones are mainly inactivated in the liver, where they are either reduced or further hydroxylated and then conjugated with glucuronic acid or sulfate for excretion (see p. 316). The reduction reactions attack oxo groups and the double bond in ring A. A combination of several inactivation reactions gives rise to many different steroid metabolites that have lost most of their hormonal activity. Finally, they are excreted with the urine and also partly via the bile. Evidence of steroids and steroid metabolites in the urine is used to investigate the hormone metabolism.
Further information
Congenital defects in the biosynthesis of steroid hormones can lead to severe developmental disturbances. In the adrenogenital syndrome (AGS), which is relatively common, there is usually a defect in 21-hydroxylase, which is needed for synthesis of cortisol and aldosterone from progesterone. Reduced synthesis of this hormone leads to increased formation of testosterone, resulting in masculinization of female fetuses. With early diagnosis, this condition can be avoided by providing the mother with hormone treatment before birth.
|
|
|
|
|
|
Lipophilic hormones |
377 |
|||
A. Biosynthesis of steroid hormones |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
OH |
|
|
|
|
|
|
DSHH |
|
|
|
|
|
H |
|
|
C27 |
|
|
|
|
|
|
|
|
|
|
HO |
|
|
CH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Cholesterol |
HO |
OH Calcitriol |
|
C |
D |
|
H |
||
|
|
|
|
|||||||
|
H |
|
|
|
|
A |
B |
D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
CH3 |
|
|
CH2OH |
HO |
|
|
|
|
|
H/S |
|
|
D |
I |
|
|
|
||
|
|
CO |
|
|
CO |
|
|
1 |
||
|
|
|
|
Cholesterol |
|
|
||||
|
|
|
HO |
|
OH |
|
|
|||
|
|
|
|
|
|
|
|
|
||
|
|
HYDHH |
|
|
|
|
|
|
|
|
|
HO |
|
O |
|
|
|
|
|
|
|
C21 |
Pregnenolone |
|
Cortisol |
|
H |
D |
CH3 |
|
||
|
|
|
|
|
|
|
||||
Y |
|
|
|
|
|
|
|
|||
CH3 |
|
|
CH2OH |
H |
|
CO |
H |
|||
|
D |
|
|
|
|
|
|
|||
|
|
CO HHH |
HO |
OHC |
CO |
|
C |
D |
S |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
HHHD |
|
|
|
A |
B |
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
O |
|
O |
|
|
Progesterone |
|
|
2 |
|
|
|
|
|
|
|
|
||||
|
Progesterone |
|
Aldosterone |
|
|
|
||||
|
|
|
|
|
|
|
||||
H
S
YOH
C19 |
|
H: Hydroxylation |
|
A |
|
|
D: Dehydrogenation |
H |
O |
|
Progesterone |
Y |
||
O |
|
|||
I : Isomerization |
|
|
||
|
H |
|
C D |
|
|
Y: Hydrogenation |
|
||
|
A |
A |
B |
|
|
OH |
S: Cleavage |
||
|
|
A: Aromatization |
O |
|
|
|
|
|
|
|
|
|
3 |
C18 |
|
|
|
Androstenedione |
|
|
|
|
|
HO |
Estradiol |
|
|
|
B. Inactivation of steroid hormones |
|
|
||
Oxidative cleavage |
Conjugate |
|
Oxidation |
|
|
CH2OH |
formation |
|
OH |
Oxidation |
|
|
Conjugate |
|
|
CO |
Reduction |
Hydroxylation |
formation |
|
|
|||
|
|
|
||
HO |
OH |
|
|
|
Conjugate |
|
|
|
|
formation |
|
|
HO |
|
|
|
|
|
|
O |
|
|
Conjugate formation |
|
|
|
|
||
Reduction |
Cortisol |
|
Estradiol |
|

378 Hormones
Mechanism of action
A. Mechanism of action of lipophilic hormones
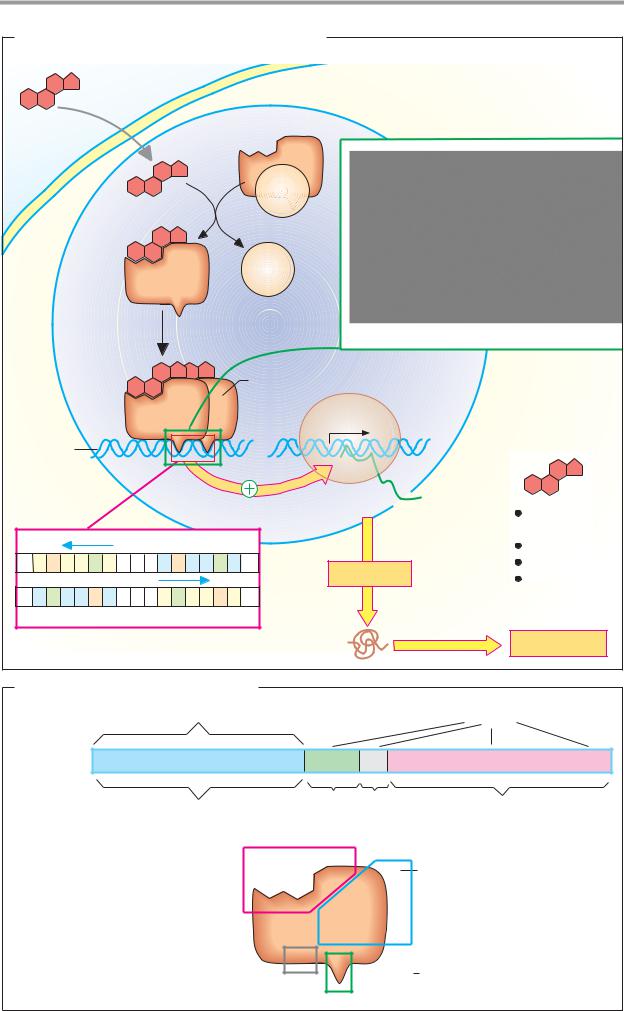
Lipophilic signaling substances include the steroid hormones, calcitriol, the iodothyronines (T3 and T4), and retinoic acid. These hormones mainly act in the nucleus of the target cells, where they regulate gene transcription in collaboration with their receptors and with the support of additional proteins (known as coactivators and mediators; see p.244). There are several effects of steroid hormones that are not mediated by transcription control. These alternative pathways for steroid effects have not yet been fully explained.
In the blood, there are a number of transport proteins for lipophilic hormones (see p. 276). Only the free hormone is able to penetrate the membrane and enter the cell. The hormone encounters its receptor in the nucleus (and sometimes also in the cytoplasm).
The receptors for lipophilic hormones are rare proteins. They occur in small numbers (103–104 molecules per cell) and show
marked specificity and high affinity for the hormone (Kd = 10–8–10–10 M). After binding
to the hormone, the steroid receptors are able to bind as homodimers or heterodimers to control elements in the promoters of specific genes, from where they can influence the transcription of the affected genes—i.e., they act as transcription factors.
The illustration shows the particularly well-investigated mechanism of action for cortisol, which is unusual to the extent that the hormone–receptor complex already arises in the cytoplasm. The free receptor is present in the cytoplasm as a monomer in complex with the chaperone hsp90 (see p. 232). Binding of cortisol to the complex leads to an allosteric conformational change in the receptor, which is then released from the hsp90 and becomes capable of DNA binding as a result of dimerization.
In the nucleus, the hormone–receptor complex binds to nucleotide sequences known as hormone response elements
(HREs). These are short palindromic DNA segments that usually promote transcription as enhancer elements (see p. 244). The illustration shows the HRE for glucocorticoids (GRE;
“n” stands for any nucleotide). Each hormone receptor only recognizes its “own” HRE and therefore only influences the transcription of genes containing that HRE. Recognition between the receptor and HRE is based on interaction between the amino acid residues in the DNA-binding domain (B) and the relevant bases in the HRE (emphasized in color in the structure illustrated).
As discussed on p.244, the hormone receptor does not interact directly with the RNA polymerase, but rather—along with other transcription factors—with a coactivator/mediator complex that processes all of the signals and passes them on to the polymerase. In this way, hormonal effects lead within a period of minutes to hours to altered levels of mRNAs for key proteins in cellular processes (“cellular response”).
B. Steroid receptors
The receptors for lipophilic signaling substances all belong to one protein superfamily. They are constructed in a modular fashion from domains with various lengths and functions. Starting from the N terminal, these are: the regulatory domain, the DNA-binding domain, a nuclear localization sequence (see p. 228), and the hormone-binding domain (see p. 73D).
The homology among receptors is particularly great in the area of the DNA-binding domain. The proteins have cysteine-rich sequences here that coordinatively bind zinc ions (A, Cys shown in yellow, Zn2+ in light blue). These centers, known as “zinc fingers” or “zinc clusters,” stabilize the domains and support their dimerization, but do not take part in DNA binding directly. As in other transcription factors (see p. 118), “recognition helices” are responsible for that.
In addition to the receptors mentioned in A, the family of steroid receptors also includes the product of the oncogene erb-A (see p. 398), the receptor for the environmental toxin dioxin, and other proteins for which a distinct hormone ligand has not been identified (known as “orphan receptors”). Several steroid receptors—e.g., the retinoic acid re- ceptor—form functional heterodimers with orphan receptors.
|
|
|
|
Lipophilic hormones |
379 |
A. Mechanism of action of lipophilic hormones |
|
|
|||
Hormone |
|
|
Target cell |
|
|
|
|
Hormone |
|
|
|
|
|
receptor |
|
|
|
|
|
hsp |
|
|
|
|
|
90 |
|
|
|
|
|
hsp |
|
|
|
|
|
90 |
|
|
|
|
|
Heat-shock |
|
|
|
Nucleus |
2x |
protein |
Glucocorticoid receptor/DNA complex |
||
|
|||||
|
|
Hormone-receptor |
DNA-binding |
|
|
|
|
domain (dimer) |
|
||
|
|
dimer |
RNA |
bound to DNA |
|
|
|
|
|
|
|
|
|
|
polymerase |
Gene |
|
DNA |
|
|
|
|
|
|
|
|
mRNA |
|
|
|
|
|
|
Steroid |
|
|
|
|
|
hormone |
|
|
|
DNA |
|
T3, T4 |
|
A G A A C A n n n |
T G T |
T C T |
Translation |
Calcitriol |
|
|
|
|
Retinoic acid |
||
T C T T G T n n n A C A A G A
Hormone response element (HRE)
|
|
Protein |
|
Cell response |
|
B. Receptors of lipophilic hormones |
|
|
|||
|
Variable length |
|
|
Domains |
|
Receptor |
A/B |
C |
D |
E |
|
gene |
|||||
|
|
|
|
||
|
Regulatory |
DNA- |
Nuclear- |
Hormone- |
|
|
domain |
binding |
targeting |
binding |
|
|
|
domain |
sequence |
domain |
|
|
|
Binds ligand |
|
Interaction with other |
|
|
|
|
|
||
|
|
|
|
nuclear components |
|
Receptor protein |
Domain E |
|
|
|
|
~250 aa |
|
|
|
||
with a total of |
|
|
Domain A/B |
||
400 – 1000 aa |
|
|
|
100 – 600 aa |
|
|
|
Domain D |
Domain C Binds to DNA |
||
|
|
~70 aa |
|
||
