
John Wiley & Sons - 2004 - Analysis of Genes and Genomes
.pdf
348 ENGINEERING PLANTS 1
of non-homologous recombination (Paszkowski et al., 1984). If the DNA also contains a marker gene, e.g. that encoding kanamycin resistance, then transformants may be selected.
(b)Biolistics. Biolistics describes the firing of small metal particles, usually tungsten or gold, coated with DNA, into target cells (Finer, Finer and Ponappa, 1999). Particle acceleration may be achieved using gun-powder, electrical discharges or pressurized gas. This method is applicable to the introduction of DNA into a wide range of cells. The fate of the DNA after it has entered the plant cell is complex. The foreign DNA is often inserted as multiple repeated copies at a particular random location within the plant genome (Pawlowski and Somers, 1998).
11.1.3Viral Vectors
Plants have many natural viral pathogens. We have seen previously how bacterial viruses have been exploited to introduce genes into bacteria (Chapter 3). Two classes of plant DNA viruses have been used as vectors to introduce foreign DNA into plants.
• Caulimovirus. Cauliflower mosaic virus (CaMV) contains an 8000 bp double-stranded circular genome that encodes eight genes (Hull and Shepherd, 1977). The genome is packaged into coat proteins, which, as for bacteriophage λ, introduces restraints on the size of recombinant genomes that can be produced that retain the ability to be infective (Figure 11.4(a)). The maximum packageable size of the viral genome has been found to be 8.3 kbp (Daubert, Shepherd and Gardner, 1983). With the removal of two non-essential viral genes, the maximum insert size is still less than 1 kbp. This greatly restricts the use of such vectors. The replication of caulimoviruses proceeds through RNA intermediates, and is consequently relatively error prone (Hull, Covey and Maule, 1987). Additionally, CaMV has a narrow host range (e.g. cauliflower, turnip and cabbage) so they can be used to introduce foreign DNA into relatively few plant species. Despite these limitations, some small genes and gene fragments have been introduced into plants using CaMV vectors (De Zoeten et al., 1989).
• Geminivirus. Geminiviruses are characterized by twin particles that encapsulate single-stranded DNA whose replication occurs in the nucleus via a double-stranded DNA intermediate, which remains infectious when cloned (Figure 11.4(b)). Monopartite geminiviruses, such as maize streak virus


350 ENGINEERING PLANTS 1
(MSV), contain a single genomic DNA, whereas bipartite geminiviruses, such as tomato golden mosaic virus (TGMV), have a segmented genome (Scholthof, Scholthof and Jackson, 1996). Geminivirus replication involves DNA-dependent DNA replication, rather than the relatively error-prone reverse transcription process employed by caulimoviruses. This led to the speculation that geminiviruses could serve as relatively stable transient gene expression vectors – which is particularly important since they infect many crop plants (Stanley, 1993). Geminiviruses, however, tend to undergo high levels of gene rearrangement and deletion during their infective cycle, which is highly undesirable for a cloning vector.
The vast majority of plant viruses possess RNA genomes and are therefore difficult to manipulate using standard techniques. However, the isolation of a full-length cDNA clone corresponding to entire RNA genomes (Ahlquist and Janda, 1984) has permitted the manipulation of the genome. Subsequent transcription of the genome in vitro using bacterial RNA polymerases allows for the reintroduction of the modified RNA genome into plant cells. For example, tobacco mosaic virus (Figure 11.4(c)) and potato virus X have both been modified to allow the introduction of foreign genes into plants (Zaitlin and Palukaitis, 2000).
11.1.4Chloroplast Transformation
In recent years, chloroplast transformation has emerged as a serious alternative to nuclear transformation (Figure 11.5). The chloroplast, like the mitochondrion, possesses its own genome. The chloroplast genome is a single circular double-stranded DNA molecule, which, in the model dicotyledonous plant Arabidopsis thaliana, is composed of approximately 155 kbp of DNA containing 87 protein coding genes (Sato et al., 1999). Chloroplast genes, like those of bacteria, are generally arranged into operons. This allows for the insertion of multiple foreign genes into the chloroplast that can be expressed from the same polycistronic transcript. The chloroplast is highly polyploid – each chloroplast may contain multiple copies of the genome – and photosynthetic cells may contain many thousands of chloroplasts. Consequently, the expression of genes inserted into the chloroplast can greatly exceed that from a gene in the nucleus. Importantly, however, the chloroplast genome is not found in pollen. Thus, the chances of spreading a foreign gene inserted into the chloroplast genome during crosses are remote.
Unlike all of the previous methods we have looked at for the transfer of genes into plants, the insertion of DNA into the chloroplast genome occurs via

11.1 CLONING IN PLANTS |
351 |
|
|
Figure 11.5. Chloroplast transformation. (A) Small leaf sections are biolistically bombarded with DNA fragments containing the aadA spectinomycin-resistance gene flanked by regions of homology to the chloroplast genome at both the 5 - and 3 -ends. (B) After four weeks on a medium containing spectinomycin, the leaf discs are bleached due to the inhibition of chloroplast protein synthesis. Successfully transformed chloroplasts initially grow as undifferentiated green calluses, which will, in the presence of appropriate growth hormones, produce shoots. (C) Spectinomycin-positive leaf segments are exposed to spectinomycin and streptomycin. The presence of the aadA gene confers broad-spectrum antibiotic resistance. Both antibiotics are therefore used to ensure that spontaneous spectinomycin-resistance mutants have not arisen. The three leaf fragments on the right-hand side of the plate have become bleached in the presence of both antibiotics because they are spontaneous spectinomycin resistance. (D) After several rounds of regeneration and screening, homogenous transgenic plantlets can be produced for the production of plants. This image was kindly provided by Professor Ralf Bock (Universitat¨ Munster,¨ Germany), and is reproduced from Bock (2001)
homologous recombination at precise locations. The foreign gene that is being introduced into the chloroplast must be flanked by sequences homologous to the chloroplast genome itself (Staub and Maliga, 1992). To obtain chloroplast transformants at a reasonable frequency, the foreign gene must be flanked by greater than 400 bp of homologous sequence at both its 5 - and 3 -ends. Chloroplast genes are themselves transcribed by chloroplast-specific promoters and use chloroplast-specific termination signals. Therefore, foreign genes to be expressed in the chloroplast must be placed between suitable promoter and terminator sequences. Additionally, a selectable marker is used to identify transformed cells. The chloroplast-specific antibiotic resistance marker aadA, conferring resistance to aminoglycoside type antibiotics such as spectinomycin, is commonly used (Goldschmidt-Clermont, 1991). Spectinomycin is a prokaryotic

352 ENGINEERING PLANTS 1
translational inhibitor that inhibits protein production in the chloroplast, but has little effect on plant cells themselves. The kanamycin-resistance gene may also be used, but alternative selectable markers to antibiotic-resistance genes are desirable as there is a risk of transferring antibiotic resistance from the plant to bacteria either in the soil or in the guts of animals that eat the plant. One selection method that does not require antibiotic selection utilizes the betaine aldehyde dehydrogenase (BADH) gene. This gene produces an enzyme that converts betaine aldehyde, a toxic compound, into glycine betaine, a non-toxic derivative, and is an effective selectable marker (Daniell, Muthukumar and Lee, 2001). Alternatively, selectable markers may be selectively removed after the construction of the transgenic plant (Iamtham and Day, 2000). For example, the marker can be flanked by loxP sequences and eliminated from the genome using Cre-mediated recombination (Corneille et al., 2001).
The double membrane of the chloroplast contained within plant cells would appear to be a serious physical barrier to the introduction of foreign DNA. However, biolistic methods (see above) are capable of delivering DNA into them (Svab, Hajdukiewicz and Maliga, 1990). Chloroplast transformation in this way results the transformation of only one, or a few, genome copies within a single plant cell. This produces genetically unstable cells containing a mixture of transformed and wild-type chloroplast genomes. Strong selective pressure (high antibiotic levels) must be applied for two to four cycles of plant regeneration to ensure that cells contain uniform recombinant chloroplasts (Figure 11.6). This is, however, a time consuming process and imposes limits on the speed at which transformants may be generated.
A range of proteins have been produced as a result of chloroplast transformation. These include proteins resulting in insect resistance herbicide resistance and drought tolerance. In addition, human therapeutic proteins can be produced in this way. For example, human somatotropin (a growth hormone that is used to treat pituitary dwarfism) has been expressed in tobacco chloroplasts (Staub et al., 2000). The protein accumulates at high levels and is both correctly folded and possesses appropriate disulphide bonds. An extremely attractive extension of this work would be to produce therapeutic proteins in edible plants. Even if therapeutic proteins can be produced in this way, mechanisms by which they can be ingested and maintained in an active form still need to be established. It should also be possible to express recombinant proteins in potato and tomato chloroplasts. For example, tomato plants have been produced expressing the aadA marker gene, where the recombinant protein accumulates in both fruits and leaves (Ruf et al., 2001).
One unique aspect of chloroplast engineering is the possibility of using operons to express multiple transgenes. In the plant nuclear genome this is not

11.1 CLONING IN PLANTS |
353 |
|
|
Primary chloroplast transformation
Cell (and chloroplast) divisions under strong 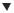 selection
selection
Further rounds of regeneration 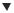 and selection
and selection
Figure 11.6. Selection of homogenous transgenic chloroplasts. Plant cells can contain between 50 and 100 chloroplasts, and each of these contains 10–20 nucleoids. Each nucleoid contains between five and 10 chloroplast genomes. Therefore, each plant cell may contain >10 000 chloroplast genomes. Transformation is likely to result in the alteration of just one of these genomes. A non-homogenous genome content in a chloroplast appears to be unstable, so multiple rounds of regeneration and selection are required to produce a homogenous population of transgenic chloroplasts (often termed homoplasmic) within the plant cell. Adapted from Bock (2001)
possible, because each gene produces a separate mRNA. Therefore, multiple transgene expression relies on either the crossing of plants containing single transgenes, or the concurrent transfer of transgenes (Daniell and Dhingra, 2002). In the chloroplast, most genes are transcribed as polycistronic messages, therefore multiple foreign genes may be expressed within the same transgene.

354 ENGINEERING PLANTS 1
For example, tobacco chloroplasts have been engineered to express three genes of the Bacillus thuringiensis cry operon (De Cosa et al., 2001). We will discuss the use of the Bacillus thuringiensis cry system below, but this approach results in very high levels of insecticidal protein accumulation – over 45% total soluble protein – with potent effects on insect pests. The lack of transfer of either the gene or its lethal protein product into pollen eliminates the danger of harming non-target and beneficial insects. Chloroplast engineering therefore appears to be a safe and environmentally friendly alternative to nuclear gene transfer for the plant biotechnology industry (Maliga, 2002).
11.2Commercial Exploitation of Plant Transgenics
For many thousands of years attempts of been made to improve crop plants. Selective breeding programmes have been used to generate varieties yielding better nutritional qualities, higher yields, or improvements that can aid cultivation and harvesting of the crop. Genetic engineering does, however, provide the opportunity to alter the properties of a plant in a directed fashion. Some examples of commercially released genetically altered plants are listed in Table 11.1.
11.2.1Delayed Ripening
Ripening is an important part of the commercial production of fruits. Fruits that are either underor over-ripened are less attractive to consumers. However, the
Table 11.1. Some commercial releases of transgenic plants, from Birch (1997)
Crop and |
Name |
Company |
Novel properties |
release date |
|
|
|
|
|
|
|
Tomato (1994) |
Flavr Savr |
Calgene |
Vine-ripened flavour, increased |
|
|
|
shelf life |
Tomato (1995) |
|
Zeneca |
Consistency of tomato paste |
Soybean |
|
Monsanto |
Glyphosate herbicide resistance |
Canola (rape) |
Roundup Ready |
|
|
Cotton (1995 – 96) |
|
|
|
Squash (1995) |
Freedom III |
Upjohn |
Multiple virus resistance |
Cotton |
Bollgard |
Monsanto |
Bacillus thuringiensis toxin for |
Potato |
NewLeaf |
|
insect resistance |
Maize (1996 – 97) |
YieldGuard |
|
|
|
|
|
|
11.2 COMMERCIAL EXPLOITATION OF PLANT TRANSGENICS |
355 |
|
|
transportation of many fully ripened soft fruits may result in their damage. This is particularly relevant to the transportation of tomatoes, where any damage can make the fruit unsellable. The protein products of a number of genes control the ripening process. One of these, encoding the enzyme polygalacturonase, is involved in the slow break-down of the polygalacturonic acid component of cell walls in the fruit pericarp. Its effects result in a gradual softening that makes the fruit edible. However, the longer the enzyme is able to act on the cell walls, the softer and more over-ripe fruit will become. Therefore, if the effects of the enzyme can be delayed then the fruit will ripen more slowly and, as a result, tomatoes can be left on the plant for longer to accumulate greater flavour. Tomatoes have been engineered so that they express less of the polygalacturonase enzyme. This was achieved through the insertion of the antisense sequence to a 5 -region of the polygalacturonase gene into the tomato genome. Expression of the antisense sequence was driven from the cauliflower mosaic virus 35S promoter, and the construct was inserted into tomato cells using Agrobacterium (Smith et al., 1988). The resulting transgenic tomatoes expressed reduced levels (6 per cent) of the polygalacturonase gene in comparison to their wild-type counterparts, and the fruit could be stored for prolonged periods before beginning to spoil. Antisense RNA has also been used to reduce the expression of some of the genes required for ethylene synthesis, again resulting in delayed ripening (Fray and Grierson, 1993).
11.2.2Insecticidal Resistance
Crop plants have been engineered to express the insecticidal toxin gene of Bacillus thuringiensis so that insects attempting to eat these plants are killed. Bacillus thuringiensis is a Gram-positive spore-forming bacterium that synthesizes a large cytoplasmic crystal containing insecticidal toxins. Different strains of the bacterium produce toxins that are effective against different insect species. The crystals are aggregates of a 130 kDa protein (Figure 11.7) that is produced as a protoxin that must be activated before it has any effect. The crystal protein is highly insoluble so it is relatively safe to humans, higher animals and most insects. However, the protein is solubilized in reducing conditions at high pH (>9.5) – the conditions commonly found in the mid-gut of lepidopteran larvae (moths and butterflies). Once it has been solubilized in the insect gut, the protoxin is cleaved by a gut protease to produce an active toxin, termed δ-endotoxin, of about 60 kDa. It binds to the midgut epithelial cells, creating pores in the cell membranes and leading to equilibration of ions. As a result, the gut is rapidly immobilized, the epithelial cells lyse, the larva stops feeding, and the gut pH is lowered by equilibration with the blood pH. This lower pH

356 ENGINEERING PLANTS 1
Figure 11.7. The structure of the Bacillus thuringiensis δ-endotoxin (Li, Carroll and Ellar, 1991)
enables the bacterial spores to germinate, and the bacterium can then invade the host, causing a lethal septicaemia. Several crops have been engineered to contain a copy of the Bacillus thuringiensis cry1Ac gene, encoding the protoxin (Table 11.1). In addition, the gene has been expressed at very high levels in the chloroplasts of tomato plants, resulting plants that are resistant to a range of insect pests (McBride et al., 1995). This approach is highly successful, but has the potential disadvantage that continuous exposure of insects to the toxin will select for the development of toxin resistance.
11.2.3Herbicidal Resistance
Several crops have been engineered for resistance to herbicides such as glyphosate (Figure 11.8). Glyphosate is an inhibitor of aromatic amino acid production in both plants and bacteria. Two approaches have been used to engineer resistance so that the herbicide can be used for weed control without damaging the crop. In the first, the target protein of the herbicide (EPSPSase) can be over-produced so that resistance occurs as a consequence of having more enzyme available to the cell (Shah et al., 1986). A second approach results from the expression of a mutant version of EPSPSase that is resistant to the herbicide within cells (Stalker, Hiatt and Comai, et al., 1985). Glyphosate-resistant

11.2 COMMERCIAL EXPLOITATION OF PLANT TRANSGENICS |
357 |
|
|
(a)OH
HO |
OH |
PN H
O O
(b)
Figure 11.8. The structure of the herbicide glyphosate. (a) Glyphosate, N-phosphono- methyl glycine, is sold as an isopropylamine salt under the trade name Roundup. It is an inhibitor of aromatic amino acid synthesis, specifically inhibiting 5-enolpyruvylshikimate- 3-phosphate synthase (EPSPSase). The herbicide is absorbed by foliage, but rapidly moves to apical areas of the plant and inhibits protein synthesis. Plants stop growing and tissues slowly degrade due to lack of proteins. Death ultimately results from dehydration and desiccation. (b) The interaction between glyphosate and EPSPSase. The active site of the enzyme is located in an interdomain cleft in the two-domain enzyme. Glyphosate (blue) binds close to shikimate-3-phosphate (red) and appears to occupy the binding site of phosphoenol pyruvate, the second substrate of EPSPase, thereby mimicking an intermediate state of the ternary enzyme–substrate complex (Schonbrunn¨ et al., 2001)
crops, e.g. the ‘Roundup Ready’ crop plants marketed by Monsanto, have the advantage of increased yield due to the elimination of weeds. However, the potentially detrimental effects of increased herbicide usage, and the potential for transmission of the herbicide-resistance gene to other plant species, are still relatively unknown (Gressel, 2000). Of course, it is easy to level criticism at agrochemical companies that produce both the herbicide and crops that are resistant to it. The potential for conflicts of inrest in a relatively captive market have led to accusations that farming interests and practices are not being served by the introduction of genetically modified crops.
11.2.4Viral Resistance
Viral damage to crop plants is responsible for huge commercial losses. The control of viral infections is based on the observations of resistance to superinfection – the infection of a plant with one viral strain protects the plant from
