
Laser-Tissue Interactions Fundamentals and Applications - Markolf H. Niemz
.pdf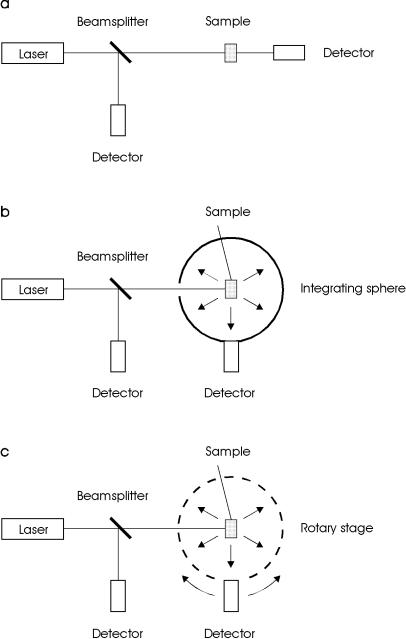
38 2. Light and Matter
Fig. 2.15. (a) Experimental setup for measuring total attenuation. (b) Experimental setup for measuring absorption. (c) Experimental setup for measuring the angular dependence of scattering
2.6 Measurement of Optical Tissue Properties |
39 |
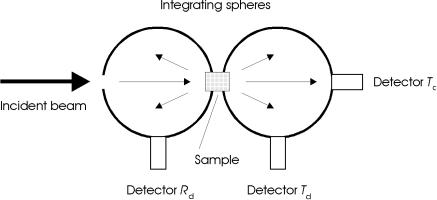
The main disadvantage of the techniques shown in Fig. 2.15a–c is that they cannot be performed simultaneously. However, it is well known that the optical properties of biological tissue are altered during heating which is often associated with exposure to laser radiation. Hence, it is more accurate to measure these properties in the same experimental arrangement and at the same time. One commonly used setup satisfying this requirement is the double-integrating sphere geometry shown in Fig. 2.16. It was first applied to the measurement of optical tissue properties by Derbyshire et al. (1990) and Rol et al. (1990), and has meanwhile turned into an unsurpassed diagnostic tool.
Fig. 2.16. Double-integrating sphere geometry for the simultaneous measurement of di erent optical tissue properties. The detectors measure the transmitted coherent intensity (Tc), the transmitted di use intensity (Td), and the reflected di use intensity (Rd)
The double-integrating sphere geometry incorporates two more or less identical spheres which are located in front and behind the sample to be investigated. One sphere integrates all radiation which is either reflected or scattered backwards from the sample. Transmitted and forward scattered radiation is absorbed in the second sphere. With three detectors, all required measurements can be performed simultaneously. In Fig. 2.16, these detectors are labeled Tc for coherent transmittance, Td for di use transmittance, and Rd for di use reflectance, respectively. Specular reflection can again be avoided by placing ba es between the sample and each detector. However, a small fraction of light in either sphere might penetrate again through the sample, thereby reaching the other sphere. The possibility of such an e ect was first recognized by Pickering et al. (1992) who then developed an improved model by taking the multiple exchange of light between both spheres into account. First results regarding optical tissue properties with this more accurate theory were published by Pickering et al. (1993).

40 2. Light and Matter
Since the Kubelka–Munk theory is one of the most frequently used methods to obtain data on optical tissue properties, we shall briefly discuss how the Kubelka–Munk coe cients introduced in Sect. 2.5 relate to measured values of di use reflectance and transmission. According to Kottler (1960), these expressions are given by
Rd = |
|
sinh(SKMyD) |
, |
(2.46) |
|
|
xcosh(SKMyD) + y sinh(SKMyD) |
||||
Td = |
|
y |
, |
(2.47) |
|
|
|
||||
xcosh(SKMyD) + y sinh(SKMyD) |
|||||
where D is the optical thickness of the slab to be considered, and SKM is the Kubelka–Munk coe cient for scattering. The parameters x and y can be expressed in terms of
1 + R2 −T2
x = d d , (2.48) 2Rd
y = x2 −1 . (2.49)
According to Kottler (1960), the convenience of applying the Kubelka–Munk method arises from the fact that scattering and absorption coe cients may be directly calculated from measured reflection and transmission coe cients, i.e. by means of
SKM = |
1 |
ln |
1 −Rd(x −y) |
, |
(2.50) |
|
yD |
T |
|||||
|
|
|
|
|||
|
|
|
d |
|
|
|
AKM = (x −1)SKM . |
|
(2.51) |
||||
The simplicity of the Kubelka–Munk model has made it a popular method for measuring optical properties of matter. In general, the model is based on the propagation of a uniform and di use radiance in a one-dimensional geometry. This is equivalent to the requirement of a forward and backward peaked phase function. Unfortunately, though, the assumptions of a purely di use radiance and a one-dimensional geometry are not satisfied by biological tissues. Thus, several attempts have been made to extend the Kubelka– Munk theory to partially collimated radiance, e.g. by Kottler (1960) and van Gemert et al. (1987), and to the case of anisotropic scattering, e.g. by Meador and Weaver (1979) and Jacques and Prahl (1987a). Despite these improvements, this method remains a rather simple approximation for the propagation of laser light in biological tissues.
Nearly all optical properties can be separated into either transport coefficients (α, αs, g) or Kubelka–Munk coe cients (AKM, SKM), depending on the theory used to obtain them. It is not surprising that transport properties are derived from theories based on the transport equation, i.e. (2.36), whereas Kubelka–Munk properties are calculated from (2.50) and (2.51).
2.6 Measurement of Optical Tissue Properties |
41 |
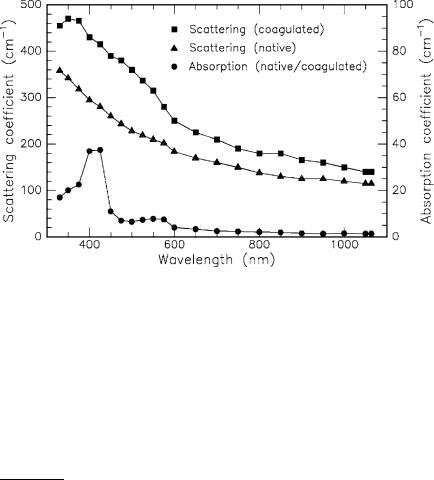
Before providing a detailed list of optical tissue parameters, it should be noted that absorption and scattering coe cients may change during laser exposure. When discussing thermal e ects in Sect. 3.2, we will find that carbonization, in particular, leads to increased absorption. However, the occurrence of carbonization is usually avoided during any kind of clinical surgery. Scattering, on the other hand, is already a ected at lower temperatures, e.g. when tissue is coagulated7. In Fig. 2.17, the absorption and scattering coe cients are shown for white matter of human brain as calculated by Roggan et al. (1995a) using the Kubelka–Munk theory. Coagulation was achieved by keeping the tissue in a bath of hot water at approximately 75◦C.
Fig. 2.17. Optical properties of white matter of human brain in its native and coagulated state. Data according to Roggan et al. (1995a)
We have now discussed di erent theoretical approaches and experimental arrangements for determining optical tissue properties. It is important to realize that both theory and experiment are necessary in order to yield valuable results. During the past decade, several research groups have been investigating all kinds of biological tissue. A short summary of the most significant results is given in Table 2.3. Type of tissue, laser wavelength, absorption coefficient, scattering coe cient, attenuation coe cient, coe cient of anisotropy, reference, and the applied theoretical method are listed. Additional data can be found in a review paper published by Cheong et al. (1990).
7Coagulation is a thermal e ect which is very useful for many types of surgery, especially in laser-induced interstitial thermotherapy (LITT), ophthalmology, and angioplasty. Further information is given in the Sects. 3.2, 4.1, and 4.6.
42 |
2. Light and Matter |
|
|
|
|
||
Table 2.3. Optical properties of human tissues in vitro |
|
||||||
|
|
|
|
|
|
|
|
Tissue |
|
λ |
α |
αs |
αt |
g |
Reference |
|
|
(nm) (cm−1) (cm−1) (cm−1) |
|
|
|||
|
|
|
|
|
|
|
|
Aorta advent. |
476 |
18.1 |
267 |
285 |
0.74 |
Keijzer et al. (1989)c |
|
Aorta advent. |
580 |
11.3 |
217 |
228 |
0.77 |
Keijzer et al. (1989)c |
|
Aorta advent. |
600 |
6.1 |
211 |
217 |
0.78 |
Keijzer et al. (1989)c |
|
Aorta advent. |
633 |
5.8 |
195 |
201 |
0.81 |
Keijzer et al. (1989)c |
|
Aorta intima |
476 |
14.8 |
237 |
252 |
0.81 |
Keijzer et al. (1989)c |
|
Aorta intima |
580 |
8.9 |
183 |
192 |
0.81 |
Keijzer et al. (1989)c |
|
Aorta intima |
600 |
4.0 |
178 |
182 |
0.81 |
Keijzer et al. (1989)c |
|
Aorta intima |
633 |
3.6 |
171 |
175 |
0.85 |
Keijzer et al. (1989)c |
|
Aorta media |
476 |
7.3 |
410 |
417 |
0.89 |
Keijzer et al. (1989)c |
|
Aorta media |
580 |
4.8 |
331 |
336 |
0.90 |
Keijzer et al. (1989)c |
|
Aorta media |
600 |
2.5 |
323 |
326 |
0.89 |
Keijzer et al. (1989)c |
|
Aorta media |
633 |
2.3 |
310 |
312 |
0.90 |
Keijzer et al. (1989)c |
|
Bladder |
|
633 |
1.4 |
88.0 |
89.4 |
0.96 |
Cheong et al. (1987)b |
Bladder |
|
633 |
1.4 |
29.3 |
30.7 |
0.91 |
Splinter et al. (1989)b |
Blood |
|
665 |
1.3 |
1246 |
1247 |
0.99 |
Reynolds et al. (1976)a |
Blood |
|
685 |
2.65 |
1413 |
1416 |
0.99 |
Pedersen et al. (1976)c |
Blood |
|
960 |
2.84 |
505 |
508 |
0.99 |
Reynolds et al. (1976)a |
Bone (skull) |
488 |
1.4 |
200 |
201 |
0.87 |
Roggan et al. (1995a)d |
|
Bone (skull) |
514 |
1.3 |
190 |
191 |
0.87 |
Roggan et al. (1995a)d |
|
Bone (skull) |
1064 |
0.5 |
120 |
121 |
0.90 |
Roggan et al. (1995a)d |
|
Brain (white) |
633 |
1.58 |
51.0 |
52.6 |
0.96 |
Splinter et al. (1989)b |
|
Brain (white) |
850 |
0.8 |
140 |
141 |
0.95 |
Roggan et al. (1995a)d |
|
Brain (white) |
1064 |
0.4 |
110 |
110 |
0.95 |
Roggan et al. (1995a)d |
|
Brain (grey) |
633 |
2.63 |
60.2 |
62.8 |
0.88 |
Splinter et al. (1989)b |
|
Breast |
|
635 |
<0.2 |
395 |
395 |
− |
Marchesini et al. (1989)a |
Gallbladder |
633 |
4.0 |
182 |
186 |
0.94 |
Maitland et al. (1993)c |
|
Liver |
|
515 |
18.9 |
285 |
304 |
− |
Marchesini et al. (1989)a |
Liver |
|
635 |
2.3 |
313 |
315 |
0.68 |
Marchesini et al. (1989)a |
Liver |
|
850 |
0.3 |
200 |
200 |
0.95 |
Roggan et al. (1995a)d |
Liver |
|
1064 |
0.3 |
150 |
150 |
0.93 |
Roggan et al. (1995a)d |
Lung |
|
515 |
25.5 |
356 |
382 |
− |
Marchesini et al. (1989)a |
Lung |
|
635 |
8.1 |
324 |
332 |
0.75 |
Marchesini et al. (1989)a |
Muscle |
|
515 |
11.2 |
530 |
541 |
− |
Marchesini et al. (1989)a |
Myocardium |
1064 |
0.4 |
175 |
175 |
0.97 |
Splinter et al. (1993)d |
|
Prostate |
850 |
0.6 |
130 |
131 |
0.96 |
Roggan et al. (1995a)d |
|
Prostate |
1064 |
0.4 |
110 |
110 |
0.96 |
Roggan et al. (1995a)d |
|
Skin (white) |
633 |
2.7 |
187 |
190 |
0.81 |
Jacques et al. (1987b)c |
|
Skin (white) |
700 |
2.7 |
237 |
240 |
0.91 |
Graa et al. (1993b)d |
|
Skin (dark) |
700 |
8.1 |
229 |
237 |
0.91 |
Graa et al. (1993b)d |
|
Uterus |
|
635 |
0.35 |
394 |
394 |
0.69 |
Marchesini et al. (1989)a |
|
|
|
|
|
|
||
a Lambert’s law |
|
|
|
|
c Di usion approximation |
||
b Kubelka–Munk or 3-flux theory |
|
d Monte Carlo simulations |
|||||

2.7 Questions to Chapter 2 |
43 |
Finally, the reader is reminded that biological tissue is something very inhomogeneous and fragile. The inhomogeneity makes it di cult to transfer experimental data from one sample to another. Usually, it is taken into account by applying generous error bars. Throughout this book, however, error bars are often neglected to facilitate readability of the figures. Fragility, on the other hand, is something the experimentor is confronted with. It was pointed out by Graa et al. (1993b) that optical properties determined in vitro may di er extremely from those valid in vivo. There are several reasons to be considered. First of all, living tissue does not have the same morphologic structure as excised tissue. One typical example is corneal tissue which turns into a turbid material within a few hours after dissection. Secondly, alterations are induced by unavoidable deformation and handling of the tissue such as drying, freezing, or just soaking in saline. According to Cilesiz and Welch (1993), dehydration especially leads to a gross e ect on the optical properties of tissue8. During all these processes, scattering is extremely enhanced. Therefore, published data are valid only for the documented type of tissue preparation and should not be taken for granted. When needing data for certain clinical applications, a careful analysis of the specific arrangement should be performed instead of blindly relying on measurements reported elsewhere.
2.7 Questions to Chapter 2
Q2.1. A laser beam is refracted on its transition from air to corneal tissue.
The angle of incidence is θ. Which angle θ is a potential angle of refraction? A: θ < θ. B: θ = θ. C: θ > θ.
Q2.2. A biological tissue has the optical albedo a = 0.9. Its coe cients of absorption and scattering are α and αs, respectively. Which is correct?
A: α = 0.9αs. B: α = 9αs. C: αs = 9α.
Q2.3. The di usion approximation may be applied to a biological tissue with an optical albedo
A: a = 0.05. B: a = 0.5. C: a = 0.95.
Q2.4. Lambert’s law describes the loss in intensity due to A: absorption. B: scattering. C: total attenuation.
Q2.5. If g is the coe cient of anisotropy, then isotropic scattering is characterized by
A: g = 0. B: g = 0.5. C: g = 1.
Q2.6. The index of refraction is approximately 1.5 for most glasses at visible wavelengths. How much light is lost due to reflection, when a red laser beam accidentally exits a laboratory through an observation window?
Q2.7. A 1mm thick optical filter has an absorption coe cient of 10cm−1 for a He-Ne laser at a wavelength of 633nm. A collinear 5mW beam is attenuated
8Theoretical and experimental results regarding the optical response of laserirradiated tissue are summarized in the excellent book edited by Welch and van Gemert (1995).
44 2. Light and Matter
by the filter with the beam propagation being perpendicular to the filter surface. What is the intensity of the attenuated laser beam when assuming that attenuation is due to absorption only?
Q2.8. Compare the intensities of scattered light in Rayleigh scattering for a 1W Nd:YAG laser at a wavelength of 1064nm and a frequency-doubled 1W Nd:YAG laser at a wavelength of 532nm.
Q2.9. The aortic wall has an absorption coe cient of 2.3cm−1 and a scattering coe cient of 310cm−1 for a He-Ne laser at a wavelength of 633nm. What is the optical albedo of the tissue?
Q2.10. When cooking an egg “sunny side up” in a pan, the egg white turns from transparent to white at a temperature of approximately 60◦C. Why?

3. Interaction Mechanisms
The variety of interaction mechanisms that may occur when applying laser light to biological tissue is manifold. Specific tissue characteristics as well as laser parameters contribute to this diversity. Most important among optical tissue properties are the coe cients of reflection, absorption, and scattering which were discussed in detail in the preceding chapter. Together, they determine the total transmission of the tissue at a certain wavelength. Thermal tissue properties – such as heat conduction and heat capacity – will be added in this chapter. On the other hand, the following parameters are given by the laser radiation itself: wavelength, exposure time, applied energy, focal spot size, energy density, and power density1. Among these, the exposure time is a very crucial parameter when selecting a certain type of interaction, as we will find later on.
During the first decades after the invention of the laser by Maiman (1960), many studies were conducted investigating potential interaction e ects by using all types of laser systems and tissue targets. Although the number of possible combinations for the experimental parameters is unlimited, mainly five categories of interaction types are classified today. These are photochemical interactions, thermal interactions, photoablation, plasma-induced ablation, and photodisruption. Each of these interaction mechanisms will be thoroughly discussed in this chapter. In particular, the physical principles governing these interactions are reviewed. Emphasis is placed on microscopic mechanisms controlling various processes of laser energy conversion. Each type of interaction will be introduced by common macroscopic observations including typical experimental data and/or histology of tissue samples after laser exposure. At the end of each discussion, a comprehensive summary of the specific interaction mechanism is given.
Before going into detail, an interesting result shall be stated. All these seemingly di erent interaction types share a single common datum: the characteristic energy density ranges from approximately 1J/cm2 to 1000J/cm2.
1In the literature, the terms used for radiometric parameters such as fluence, irradiance, intensity, and energy dose are often somewhat confusing. Throughout this book, the following agreements are met: power is expressed in units of W,
energy in units of J, the synonyms power density, intensity, and irradiance in units of W/cm2, the synonyms energy density, fluence, and radiant exposure in units of J/cm2, and energy dose in units of J/cm3 (see also the Appendix).
46 3. Interaction Mechanisms
This is surprising, since the power density itself varies over 15 orders of magnitude! Thus, a single parameter distinguishes and primarily controls these processes: the duration of laser exposure which is mainly identical with the interaction time itself.
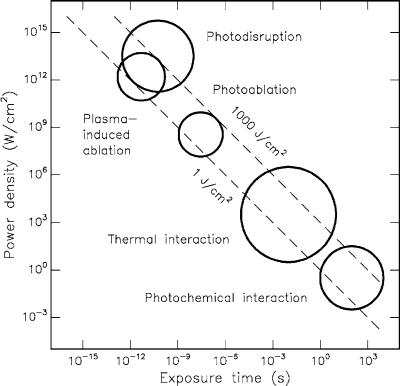
A double-logarithmic map with the five basic interaction types is shown in Fig. 3.1 as found in several experiments. The ordinate expresses the applied power density or irradiance in W/cm2. The abscissa represents the exposure time in seconds. Two diagonals show constant energy fluences at 1J/cm2 and 1000J/cm2, respectively. According to this chart, the time scale can be roughly divided into five sections: continuous wave or exposure times > 1s for photochemical interactions, 1min down to 1μs for thermal interactions, 1μs down to 1ns for photoablation, and < 1ns for plasma-induced ablation and photodisruption. The di erence between the latter two is attributed to di erent energy densities. They will be addressed separately in Sects. 3.4 and 3.5, since one of them is solely based on ionization, whereas the other is an associated but primarily mechanical e ect.
Fig. 3.1. Map of laser–tissue interactions. The circles give only a rough estimate of the associated laser parameters. Modified from Boulnois (1986)
3.1 Photochemical Interaction |
47 |
Obviously, Fig. 3.1 proves the statement from above that the total energy density relevant for medical laser applications ranges from about 1J/cm2 to 1000J/cm2. The reciprocal correlation between power density and exposure time clearly demonstrates that roughly the same energy density is required for any intended type of interaction. Thus, the exposure time appears to be the main parameter responsible for the variety of interaction mechanisms.
Adjacent interaction types cannot always be strictly separated. As shown in the following sections, thermal e ects may also play an important role during photochemical interaction. And even ultrashort laser pulses with pulse durations shorter than 100ps – each of them having no thermal e ect – may add up to a measurable increase in temperature if applied at repetition rates higher than about 10–20Hz, depending on the laser. These two examples reveal our need for a better understanding of each laser–tissue interaction. This task, of course, is aggravated due to the inhomogeneity of most tissues which cannot be altered. However, the basic physics involved in each interaction becomes accessible if enough data are collected to fit unknown parameters.
3.1 Photochemical Interaction
The group of photochemical interactions stems from empirical observations that light can induce chemical e ects and reactions within macromolecules or tissues. One of the most popular examples was created by evolution itself: the energy release due to photosynthesis. In the field of medical laser physics, photochemical interaction mechanisms play a significant role during photodynamic therapy (PDT). Frequently, biostimulation is also attributed to photochemical interactions, although this is not scientifically ascertained. After a detailed description of the physical background, both of these methods will be discussed in this section.
Photochemical interactions take place at very low power densities (typically 1W/cm2) and long exposure times ranging from seconds to continuous wave. Careful selection of laser parameters yields a radiation distribution inside the tissue that is determined by scattering. In most cases, wavelengths in the visible range (e.g. Rhodamine dye lasers at 630nm) are used because of their e ciency and their high optical penetration depths. The latter are of importance if deeper tissue structures are to be reached.
During PDT, spectrally adapted chromophores are injected into the body. Monochromatic irradiation may then trigger selective photochemical reactions, resulting in certain biological transformations. A chromophore compound which is capable of causing light-induced reactions in other nonabsorbing molecules is called a photosensitizer. After resonant excitation by laser irradiation, the photosensitizer performs several simultaneous or sequential decays which result in intramolecular transfer reactions. At the end of these diverse reaction channels, highly cytotoxic reactands are released causing an irreversible oxidation of essential cell structures. Thus, the main
