
Micro-Nano Technology for Genomics and Proteomics BioMEMs - Ozkan
.pdf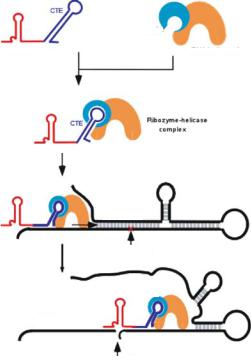
506 MAKI SHIOTA, MAKOTO MIYAGISHI AND KAZUNARI TAIRA
|
CTE |
|
|
|
Adapter |
Sliding ribozyme |
RNA helicase A |
|
|
|
|
|
|
Ribozyme-helicase |
|
CTE |
|||
|
complex |
||
|
|
Sliding ribozyme
Binding
Target mRNA
Sliding
Cleavage site
Unwinding & cleavage
Target mRNA
Cleavage site
FIGURE 17.5. Schematic representation of the way in which a CTE-Rz coupled to an RNA helicase might cleave a hidden target site upon the unwinding of local secondary structure.
an RNA helicase coupled to a ribozyme via the CTE might efficiently guide the ribozyme to its target site by resolving any inhibitory structure in the target mRNA, with the resultant efficient cleavage of the mRNA.
Figure 17.5 shows a schematic representation of the way in which a CTE-ribozyme sequence coupled to an RNA helicase might cleave a sequestered target site after the unwinding of local secondary structure. We designed a tRNAVal-ribozyme, with a pol III-mediated expression system, and attached the CTE sequence to the 3’ end. We chose the TAR region of the long terminal repeat (LTR) of HIV-1 as the target of the ribozyme [41, 51]. The TAR region does not mutate because it is essential for the replication of HIV-1. Thus, the TAR region might be an effective target in any potential gene therapy against HIV-1. However, the stem structure of the TAR region prevents access by functional RNAs, such as antisense RNAs and ribozymes. If our hybrid CTE-ribozyme could disrupt the stem structure of the TAR region, locate its target and cleave the mRNA, this ribozyme might be an effective drug against HIV-1 irrespective of any mutations in the virus.
We prepared CTE-connected and unconnected ribozymes that were targeted to the TAR region, as shown in Figure 17.6A. The target gene, which consisted of the LTR of HIV-1 and a gene for luciferase, was stably expressed in HeLa cells. Figure 17.6B shows the suppression of the activity of the LTR-driven luciferase by the CTE-ribozyme. TAR Rz4 and TAR Rz5 were designed to target sites that we predicted would be inaccessible within the
508 |
MAKI SHIOTA, MAKOTO MIYAGISHI AND KAZUNARI TAIRA |
well-documented stable stem structure of the TAR region. These ribozymes without the CTE (non-CTE ribozymes) had no effects on the levels of activity of the reporter luciferase. When the CTE was attached, these ribozymes strongly inhibited the luciferase activity (Figure 17.6B, lanes 11 and 13), with an 80% reduction in reporter activity. Furthermore, these CTE-coupled ribozymes had higher activity than non-CTE ribozymes that were designed to target “open” sites (TAR Rz1, LTR Rz2, Luc Rz3). Attachment of the CTE to other ribozymes also enhanced their activity. In particular TAR CTE-Rz4 and CTE-Rz5 had suppressive activities similar to those of TAR CTE-Rz1, LTR CTE-Rz2, and Luc CTE-Rz3. These results suggest that addition of the CTE sequence might allow all ribozymes to attack their targets efficiently.
We tested the general applicability of the CTE-Rz by targeting several endogenous targets, such as the mRNA for mouse procaspase-3 (CPP 3), and we obtained similar results [113]. It appears that CTE-ribozymes have specificity, strong activity, and general utility. However, the most important observation was that CTE-ribozyme were able to cleave their target mRNAs at any site, regardless of the predicted secondary or tertiary structure. All of our CTE-ribozymes has strong activity in cultured cells, and, in many cases, they were very active when the respective parental ribozymes, without the CTE, were inactive. Therefore, the hybrid CTE-ribozyme should have broad applicability because it is extremely easy to design and use. By contrast, previously developed ribozyme technologies require specialized skills. Furthermore, the improved efficacy of the CTE-ribozymes makes them even more suitable than earlier ribozymes for a wide range of applications, as described below. Having improved the efficacy of our ribozyme by eliminating constraints related to selection of target sites, we have produced a powerful tool both for basic research and for therapeutic interventions. We have also demonstrated that a poly(A) tail can be used instead of a CTE with similar results (Kawasaki, 2002).
17.5. MAXIZYMES: ALLOSTERICALLY CONTROLLABLE RIBOZYMES
Several years ago, we developed a completely new type of ribozyme as a result of efforts to shorten the hammerhead ribozyme. We succeeded in creating an allosteric ribozyme, the maxizyme, which functions as a dimer with significant specificity and activity both in vitro and in vivo [49–51, 101, 102]. This dimeric allosteric ribozyme allowed the first successful demonstration of an artificially created enzyme with potential utility as a biosensor not only in vitro but also in vivo (see below). As discussed below, we developed novel dimeric RNA motifs as a result of studies of “minizymes” that were aimed at shortening ribozymes. The designation “minizyme” is applied to shortened (minimized) ribozymes, but this designation also had a negative connotation as a ribozyme with extremely low activity (minimum). By contrast our novel ribozymes have extremely high activity in cells [49–51]. Therefore, we chose the name “maxizyme” for these new highly active minimized dimeric ribozymes [minimized, active, x-shaped (functions as a dimer), and intelligent (allosterically controllable) ribozyme].
17.5.1. Shortened Hammerhead Ribozymes that Function as Dimers
The cleavage of RNA by ribozymes has a general requirements for the presence of a divalent metal ion such as a magnesium ion [7, 11, 15, 18, 21, 62, 64, 93, 109–112, 119].
ENGINEERED RIBOZYMES: EFFICIENT TOOLS FOR MOLECULAR GENE THERAPY |
509 |
The catalytic domain of a hammerhead ribozyme captures these catalytically indispensable Mg2+ ions. The key to the creation of various allosteric ribozymes is the ability to control the capture of Mg2+ ions via a conformational change in the catalytic core of the ribozyme. For example, it is possible to replace RNA by the more stable DNA in the substrate-binding region or to shorten the stem-loop II region. However, for the potential application of ribozymes in a clinical setting, ease of design and economics dictate that smaller size is preferable. A smaller version of the hammerhead ribozyme, namely, a minizyme, has been made by replacing the stem-loop II region by a short linker [2, 19, 66, 106]. Unfortunately, such minizymes have quite low activity, as compared to the parental ribozymes. However, we found that a minizyme that lacked the entire stem-loop II region was essentially as active as the “wild-type” parent [2]. Kinetic and NMR analyses revealed that the shortened ribozyme was essentially inactive as a monomer but had extremely high catalytic activity as a dimer (Figure 17.7A) [47, 49]. This ribozyme, which is a “dimeric minizyme”, was renamed “maxizyme” [49–51, 101, 102].
We extended our studies of dimeric minizymes by designing a heterodimeric system composed of two different monomers, maxizyme left (MzL) and maxizyme right (MzR) [47, 50, 51]. In this system, shown in Figure 17.7B, the substrate is cleaved only when MzL and MzR form a dimer. Since such maxizymes have two substrate-binding regions, we were able to convert our heterodimeric maxizyme into an allosteric version that was able to function as a sensor.
17.5.2. Design of an Allosterically Controllable Maxizyme
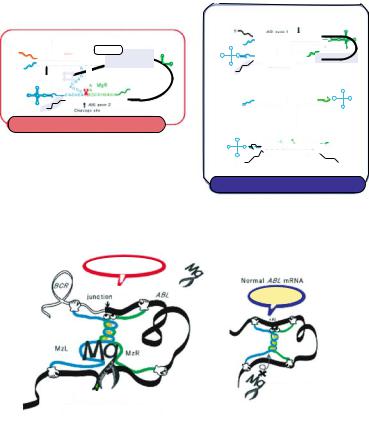
Ribozymes can target essentially any RNA at specific sites but there is a minimum required cleavable sequence: cleavage occurs only after the sequence NUX (where N is any base and X is A, C, or U) [94]. In some cases, such a cleavable triplet sequence is not available at a suitable position in the target RNA. We have focused on such a case that involves a chimeric mRNA of clinical importance. Chimeric mRNAs are generated by chromosomal translocations and they are fusion mRNAs in which the first part and the second part are derived from two different genes. They are often involved in the pathogenesis of disease. A well known example of a pathogenic chromosomal translocation is the Philadelphia chromosome, which causes chronic myelogenous leukemia (CML) [71, 77]. The Philadelphia chromosome is the result of a reciprocal translocation that involves the BCR and ABL genes, and the product of translocation is a fusion mRNA. Fusion mRNAs of this type are tumor-specific and pathogenetically important and, thus, they are obvious targets for nucleic acid therapeutics [95]. However, because of the absence of a NUX sequence near the site of fusion in the target mRNA, conventional ribozymes cannot distinguish the chimeric mRNA from the normal parental mRNAs [48, 50, 112]. Both the BCR gene and the ABL gene are important for cell survival. Thus, when we design ribozymes that might cleave the chimeric mRNA, we must be sure to avoid cleavage of normal mRNAs, which share sequences with the abnormal fusion mRNA.
There have been many attempts to cleave chimeric mRNAs specifically using ribozymes, but it is extremely difficult to cleave only the chimeric mRNA without affecting the normal parental mRNAs [29, 82]. As mentioned above, maxizymes can bind to two different target sites. We took advantage of the two substrate-binding regions to design a maxizyme with one substrate-binding region that corresponds to the abnormal junction
512 |
MAKI SHIOTA, MAKOTO MIYAGISHI AND KAZUNARI TAIRA |
cleaved the target RNA as the result of a structural change that occurred only in the presence of the oncogenic chimeric mRNA with the junction sequence [50].
We evaluated our maxizyme (MzL plus MzR) in cultured mammalian cells. To ensure the efficient formation of dimers in cells, we used the promoter of the gene for a tRNA that is recognized by RNA polymerase III, as described above. We introduced a conventional ribozyme, each individual monomer of the maxizyme, and both monomers together into cells derived from patients with CML. We demonstrated that the active maxizyme was a heterodimer and that cleavage by the heterodimeric maxizyme, rather than an antisense effect, was responsible for the specific suppression of expression of the BCR-ABL mRNA. Moreover, this maxizyme had significantly higher activity than the “wild-type” ribozyme from which it was derived.
17.5.3. Inactivation of an Oncogene in a Mouse Model
There have been many attempts, using various approaches, to construct artificial allosteric enzymes, but success in vivo has been minimal. The maxizyme was, to our knowledge, the first artifical allosteric enzyme to function in cells in culture.
We next examined the anti-tumor effect of our maxizyme in animals [101]. We used a retroviral system for the expression of the maxizyme in leukemic cells. We subcloned two tRNAVal-driven expression cassettes, which corresponded to each component of the heterodimer, in tandem in the retroviral vector. A line of CML cells (BV173) was transduced either with a control vector, in which the maxizyme sequence had been deleted, or with the maxizyme-encoding vector. We then injected a bolus of each line of transduced BV173 cells into the tail veins of mice.
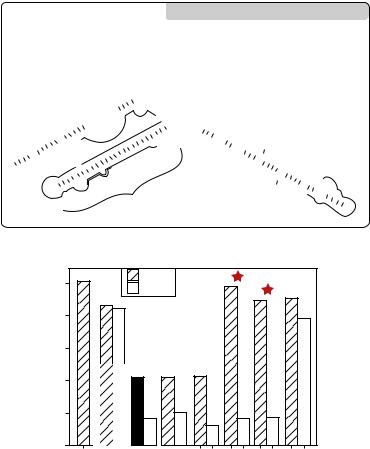
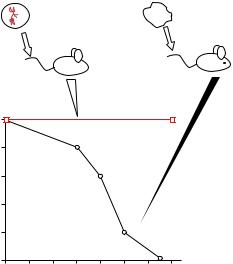
The differences between the two groups of mice were reflected in their mortality rates (Figure 17.9). All of the mice injected with control BV173 cells died of diffuse leukemia, confirmed at necropsy, after 6 to 13 weeks (median survival time, 9 weeks), whereas mice injected with maxizyme-transduced BV173 cells remained healthy. Our results indicate that each subunit of the maxizyme, introduced by the retroviral vector, was produced at the appropriate concentration to support dimerization in vivo. Also, the maxizyme apparently functioned successfully in animals, cleaving BCR-ABL mRNA with exceptional efficiency.
At present, use of kinase inhibitors [22] and allogeneic transplantation are the only effective therapies for CML, with only half of all patients, on average, being eligible for the latter treatment because of the limited availability of donors and age restrictions. Our results raise the possibility that our maxizyme might be useful for purging bone marrow cells in cases of CML treated by autologous transplantation, when it would presumably reduce the incidence of relapse by decreasing the tumorigenicity of contaminating CML cells in the transplant.
17.5.4. Generality of the Maxizyme Technology
The maxizyme is of considerable interest because it imparts a sensor function to short ribozymes that act as a dimer. Using this sensor function, we can specifically cleave abnormal chimeric mRNA exclusively, without affecting the normal mRNA, which should not be cleaved. Maxizyme technology is not limited to the disruption of the abnormal chimeric gene
ENGINEERED RIBOZYMES: EFFICIENT TOOLS FOR MOLECULAR GENE THERAPY |
513 |
Maxizyme-transduced |
Control BV173 cells |
|
BV173 cells |
||
|
||
♦ |
|
|
100 |
|
|
|
|
Maxizyme |
||
|
|
|
|
|
|
|||
(%) |
80 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Survival |
60 |
|
|
|
|
|
|
|
40 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20 |
|
|
|
|
|
Control |
|
|
0 |
|
|
|
|
|
|
|
|
0 |
2 |
4 |
6 |
8 |
10 |
12 |
14 |
Time after injection (weeks)
FIGURE 17.9. The antitumor effects of the maxizyme in a murine model of chronic myelogenous leukaemia (CML). The survival of animals was monitored daily for more than 20 weeks after inoculation; all control mice died within 13 weeks, whereas maxizyme-treated mice remained disease-free for the entire period of the investigation.
in CML. Abnormal chimeric genes generated from reciprocal chromosomal translocations are frequently found in several types of leukemia. Maxizymes have been used successfully to cleave only abnormal target mRNAs, which lack a NUX cleavage site at the junction, in the case of acute lymphoblastic leukemia (ALL) and acute promyelocytic leukemia (APL), without any damage to the products of normal genes [102]. Abnormal chimeric mRNAs are also generated by errors in splicing. A maxizyme can be designed to act against each possible transcript and should be able to distinguish its target specifically from other mRNAs. We have already constructed various maxizymes that target different chimeric genes, and each of them is very active and highly specific [50, 102]. Thus, maxizymes appear to be powerful gene-inactivating agents with allosteric functions that allow them to cleave any type of chimeric mRNA specifically. To our knowledge, the maxizyme is the first artificial, allosteric enzyme whose activity has been demonstrated at the animal level, highlighting its potential utility in a clinical setting [31, 102]. It should be noted, however, that the transcripts of chimeric genes of the type discussed here can also be destroyed by small interfering RNAs (siRNAs; [81, 91]).
17.6. IDENTIFICATION OF GENES USING HYBRID RIBOZYMES
As noted in section 4.2, we developed a hybrid ribozyme that coupled the cleavage activity of hammerhead ribozymes with the unwinding activity of RNA helicase and was able to cleave its target mRNA extremely efficiently, regardless of the secondary structure
514 |
|
|
|
|
|
|
|
|
|
|
|
|
|
MAKI SHIOTA, MAKOTO MIYAGISHI AND KAZUNARI TAIRA |
||||||||||||||
Anti-Fas |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
antibody |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Re-introduce Rz into |
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
fresh target cells |
|
|
|
|
|
|
|
|
|
||||||
|
Fas |
|
|
|
|
|
|
|
|
Rescue |
|
5 ' |
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
3 ' |
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ribozyme gene |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 ' |
|
|
|
|
|
5 |
' |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Select for |
|
|
|
|
|
|
|
ldentification of target gene |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
with pro-apoptotic function |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
desired phenotype |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Survival |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A |
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
A |
|
|
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
–– |
A |
|
|
A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
A |
|
|
|
A |
|
–– |
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Ribozyme - A 60 library
Apoptosis
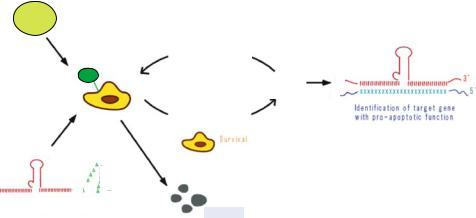
FIGURE 17.10. Schematic diagram of the application of the gene discovery system to the Fas-induced apoptosis using libraries of poly(A)-connected hybrid-Rz. Hela-Fas cells that expressed the randomized Rz-60 libraries were treated with the Fas-specific (Anti-Fas) antibodies.
of the RNA. We attempted to use this novel ribozyme not only to cleave specific known target mRNAs but also to identify genes associated with specific phenotypes in cells. This can be accomplished using ribozymes with randomized binding arms, as has been done with hairpin ribozyme [5, 44, 61, 114]. The sequence of the human genome has become available, and it will be extremely valuable to have methods for the rapid identification of important genes. We conducted this function analysis by using hybrid ribozymes, which coupled cleavage activity with the unwinding activity of an endogenous RNA helicase [100]. We demonstrate that ribozyme of this type are able to cleave the target mRNA at a chosen site, regardless of the putative secondary or tertiary structure in the vicinity of the target site, and thus they can be used for rapid identification of functional genes in the post-genome era.
We attached a poly(A) 60 sequence to the 3’ end of a tRNAVal-driven ribozyme (RzA60) instead of the CTE sequence [75, 113]. This poly(A) sequence interacts with endogenous RNA helicase elF4AI via interactions with poly(A)-binding protein (PABP) and PABP-interacting protein-1 (PAIP). We demonstrated that this complex was able to unwind an RNA duplex substrate effectively, and cleaved otherwise inaccessible target sites. We used these ribozymes to discover the functions of unknown genes. Since our hybrid ribozymes can attack structured sites, they can attack mRNAs with high-level efficiency. If libraries of hybrid ribozymes with randomized binding arms are introduced into cells, the genes associated with any changes in phenotype can be readily identified by sequencing the specific ribozyme clone [100]. Figure 17.10 shows a schematic representation of our gene-discovery system.
We established a novel system for screening functional genes in the signaling pathway of Fas-induced apoptosis in HeLa-Fas cells using randomized Rz-A60-expression libraries. In this system, we randomized 10 nt in each substrate-binding arm of Rz-A60, and then retroviral vectors that carried the randomized Rz-A60-expression libraries were introduced
ENGINEERED RIBOZYMES: EFFICIENT TOOLS FOR MOLECULAR GENE THERAPY |
515 |
into HeLa-Fas cells. After treatment of these HeLa-Fas cells with Fas-specific antibodies, which normally induces apoptosis, cells that survived were collected and the genomic DNA was isolated from each clone. Sequencing of the randomized region of Rz-A60 in each genomic DNA enabled us rapidly to identify genes involved in the Fas-induced apoptotic pathway.
We identified a variety of genes with pro-apoptotic functions, such as genes for FADD, caspase 8, caspase 9 and caspase 3. In the absence of the poly(A) tail, we would not have identified genes for FADD and caspase 8 in our first screening with the randomized Rz libraries, since only poly(A)-connected ribozymes targeted to FADD mRNA or caspase 8 mRNA affected the expression of target genes. Our study demonstrated the successful application of a hybrid Rz to gene discovery. Using this gene discovery system, we have also identified many factors [76] involved in other apoptotic pathways [36–38], metastasis [98, 99], Alzheimer’s disease [79, 80], and the roles of microRNAs [56].
17.7. SUMMARY AND PROSPECTS
As discussed above, it is now possible to achieve high-level activity of ribozymes in vivo and to cleave any specific target RNA in the cell using either our maxizyme or a hybrid ribozyme. These ribozymes also efficient tools for the analysis of gene function.
Our efficient ribozyme-expression systems are also applicable to the expression of small interfering RNAs (siRNAs), which induce the sequence-dependent degradation of a cognate mRNA via RNA interference (RNAi). The application of RNAi in mammals has the potential to allow the systematic analysis of gene expression and also the therapeutic silencing of gene expression [67].
Since siRNAs are 21to 23-nt RNA duplexes with 2- or 3-nt overhanging 3’ ends, the pol III system, which is suitable for efficient transcription of small RNAs, is also useful for the expression of siRNAs. Another advantage of the pol III system is that transcription terminates at four or more T residues, leaving 1-4 U residues at the 3’ terminus of the nascent RNA. These properties allow use of DNA templates to synthesize small RNAs with structural features close to those of siRNAs that are active in vivo.
A number of groups have developed plasmid-based siRNA-expression systems using pol III promoters [9, 59, 69, 83, 85, 96, 118]. Two pol III promoters have been used predominantly, the U6 promoter and the H1 promoter. As mentioned above, tRNAVal- driven ribozymes are transcribed at high levels, and the transcripts are transported to the cytoplasm without exception when an appropriate linker is used. Our work indicates that RNAi in mammalian cells occurs in the cytoplasm [39]. Therefore, the tRNAVal expression system should also be ideal for the expression of siRNA. In fact, tRNA-dsRNAs, in which a short hairpin structure is attached to a tRNA-like ribozyme, is efficiently transported to the cytoplasm, and effectively induces RNAi-mediated gene silencing (Kuwabara, 2003). tRNA-dsRNAs should be powerful tools for studies of the functions of genes in mammalian cells and they might also be useful as therapeutic agents as ribozymes.
Now we can employ two versatile gene knock-down tools; ribozymes and siRNAs, as usage for any purpose.
