
Micro-Nano Technology for Genomics and Proteomics BioMEMs - Ozkan
.pdf436 |
PHILIP SANTANGELO ET AL. |
[128]M. Silhol, M. Tyagi, M. Giacca, B. Lebleu, and E. Vives. Eur. J. Biochem., 269:494–501, 2002.
[129]S. Violini, V. Sharma, J.L. Prior, M. Dyszlewski, and D. Piwnica-Worms. Biochemistry, 41:12652–12661, 2002.
[130]M. Belting. Trends Biochem. Sci., 28:145–151, 2003.
[131]B. Allinquant, P. Hantraye, P. Mailleux, K. Moya, C. Bouillot, and A. Prochiantz. J. Cell. Biol., 128:919–927, 1995.
[132]C.M. Troy, D. Derossi, A. Prochiantz, L.A. Greene, and M.L. Shelanski. J. Neurosci., 16:253–261, 1996.
[133]A. Astriab-Fisher, D. Sergueev, M. Fisher, B.R. Shaw, and R.L. Juliano. Pharm. Res., 19:744–754, 2002.
[134]N. Nitin, P.J. Santangelo, G. Kim, S. Nie, and G. Bao. Peptide-linked molecular beacons for efficient delivery and rapid mRNA detection in living cells, submitted to Nucleic Acids Res., 2004.
[135]Y. Nakagami, M. Ito, T. Hara, T. Inoue, and S. Matsubara. Acta. Oncol., 42:227–236, 2003.
[136]A. Urbano, R. McCaffrey, and F. Foss. J. Biol. Chem., 273:34820–34827, 1998.
[137]M.J. Fraser, S.J. Tynan, A. Papaioannou, C.M. Ireland, and S.M. Pittman. J. Cell. Sci., 109 (Pt 9):2343–2460, 1996.
[138]P. Mitchell and D. Tollervey. Nat. Struct. Biol., 7:843–846, 2000.
[139]M.J. Moore. Cell, 108:431–434, 2002.
[140]A. van Hoof and R. Parker. Cell, 99:347–350, 1999.
[141]C. Allmang, E. Petfalski, A. Podtelejnikov, M. Mann, D. Tollervey, and P. Mitchell. Genes. Dev., 13:2148– 2158, 1999.
[142]P. Mitchell, E. Petfalski, A. Shevchenko, M. Mann, and D. Tollervey. Cell, 91:457–466, 1997.
[143]T. Kadowaki, M. Hitomi, S. Chen, and A.M. Tartakoff. Mol. Biol. Cell., 5:1253–1263, 1994.
[144]N.I. Zanchin and D.S. Goldfarb. Mol. Cell. Biol., 19:1518–1525, 1999.
[145]J.S. Jacobs, A.R. Anderson, and R.P. Parker. Embo. J., 17:1497–1506, 1998.
[146]Y. Matsumoto, R. Fishel, and R.B. Wickner. Proc. Natl. Acad. Sci. U.S.A., 87:7628–7632, 1990.
[147]R.A. Deshpande and V. Shankar. Crit. Rev. Microbiol., 28:79–122, 2002.
[148]M. Irie. Pharmacol. Ther. 81:77–89, 1999.
[149]A. Egesten, K.D. Dyer, D. Batten, J.B. Domachowske, and H.F. Rosenberg. Biochim. Biophys. Acta., 1358: 255–260, 1997.
[150]B.R. Chapados, Q. Chai, D.J. Hosfield, J. Qiu, B. Shen, and J.A. Tainer. J. Mol. Biol., 307:541–556, 2001.
[151]P.S. Eder, R.Y. Walder, and J.A. Walder. Biochimie, 75:123–126, 1993.
[152]B. Rydberg and J. Game. Proc. Natl. Acad. Sci. U.S.A., 99:16654–16659, 2002.
[153]A.L. ten Asbroek, M. van Groenigen, M. Nooij, and F. Baas. Eur. J. Biochem., 269:583–592, 2002.
[154]S. Agrawal. Biochim. Biophys. Acta., 1489:53–68, 1999.
[155]A. De Mesmaeker, K.H. Altmann, A. Waldner, and S. Wendeborn. Curr. Opin. Struct. Biol., 5:343–355, 1995.
[156]M. Manoharan. Biochim. Biophys. Acta., 1489:117–130, 1999.
[157]E.A. Lesnik, C.J. Guinosso, A.M. Kawasaki, H. Sasmor, and M. Zounes et al. Biochemistry, 32:7832–7838, 1993.
[158]M. Petersen and J. Wengel. Trends Biotechnol., 21:74–81, 2003.
[159]U. Christensen, N. Jacobsen, V.K. Rajwanshi, J. Wengel, and T. Koch. Biochem. J., 354:481–484, 2001.
[160]M. Frieden, H.F. Hansen, and T. Koch. Nucleosides Nucleotides Nucleic Acids, 22:1041–1043, 2003.
[161]P.E. Nielsen, M. Egholm, R.H. Berg, and O. Buchardt. Science, 254:1497–1500, 1991.
[162]T. Ratilainen, A. Holmen, E. Tuite, P.E. Nielsen, and B. Norden. Biochemistry, 39:7781–7791, 2000.
[163]S.A. Kushon, J.P. Jordan, J.L. Seifert, H. Nielsen, P.E. Nielsen, and B.A. Armitage. J. Am. Chem. Soc., 123:10805–10813, 2001.
[164]X. Liu and W. Tan. Anal. Chem., 71:5054–5059, 1999.
[165]D. Kambhampati, P.E. Nielsen, and W. Knoll. Biosens. Bioelectron., 16:1109–1118, 2001.
[166]F.J. Steemers, J.A. Ferguson, and D.R. Walt. Nat. Biotechnol., 18:91–94, 2000.
[167]N. Hamaguchi, A. Ellington, and M. Stanton. Anal. Biochem., 294:126–131, 2001.
[168]R. Yamamoto, T. Baba, and P.K. Kumar. Genes Cells, 5:389–396, 2000.
13
Fluorescent Lanthanide Labels with Time-Resolved Fluorometry in DNA Analysis
Takuya Nishioka, Jingli Yuan, and Kazuko Matsumoto
Department of Chemistry and Advanced Research Institute for Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku-ku, Tokyo 169-8555, Japan
13.1. INTRODUCTION
Some lanthanide (Eu3+, Tb3+, Sm3+, and Dy3+) complexes are known to be luminescent. Compared with organic fluorescent compounds, the fluorescence of lanthanide chelates has several special different properties; (1) The lifetime of the lanthanide chelates is very long; that of europium(III) and terbium(III) chelates usually ranges from several hundred microseconds to more than one millisecond, and that of samarium(III) and dysprosium(III), 10 to 100 microseconds. (2) Stokes shifts are very large: the chelates are excited by UV light (310–350 nm) and emit fluorescence in the visible region. (3) The emission profiles are sharp, having a full width at half maximum (FWHM) of only 10 nm.
In the last 20 years, lanthanide chelates have been successfully developed as fluorescence labels for highly sensitive detection of various biological molecules in time-resolved fluorometry. Lanthanide fluorescence labels have been used in time-resolved fluorometry of immunoassay (TR-FIA), DNA hybridization assay, high performance liquid chromatography (HPLC), fluorescence imaging microscopy, and other bioassays, and shown great improvement of the sensitivity compared to the conventional fluorometry using organic fluorescent labels. The high sensitivity or detectability is due to easy distinction of the specific fluorescence signal of the lanthanide labels from background signals present in most biological samples. Time-resolved fluorometry also obviates the problems associated with light scattering of the optical components. These problems cannot be solved easily by the
FLUORESCENT LANTHANIDE LABELS WITH TIME-RESOLVED FLUOROMETRY |
439 |
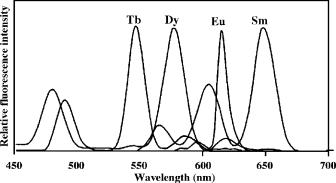
FIGURE 13.2. Emission spectra of the complexes of Eu3+ , Sm3+ , Tb3+ , and Dy3+ with PTA in the presence of 1,10-phenanthroline, Triton X-100, and Y3+ . Modified from Anal. Chim. Acta, 256, 9–16 (1992).
Since Weissman discovered in 1942 that Eu3+ complexes with β-diketone-type ligands emit fluorescence when excited with UV light [12], this class of complexes has been investigated intensively and is used for lanthanide analysis [13] and for laser materials [14]. In these early works, the researchers found that 2-naphthoyltrifluoroacetone (β-NTA), 2-thenoyltrifluoroacetone (TTA) and pivaloyltrifluoroacetone (PTA) are the best ligands for fluorescent complexes of Eu3+, Sm3+, Tb3+ and Dy3+; among these β-NTA and TTA are effective only for Eu3+ and Sm3+, whereas PTA is effective for all four ions at room temperature. Figure 13.2 shows the emission spectra of the Eu3+, Sm3+, Tb3+, and Dy3+ complexes with PTA in the presence of 1,10-phenanthroline, Triton X-100, and Y3+ [15]. However, the bidentate lanthanide-β-diketonate complexes in Figure 13.2 cannot be used as labels, since there is no active binding group on these β-diketone ligands and these complexes are not very stable with the stability constants only in the order of 103 to 106 [16, 17], therefore the complexes dissociate in highly diluted solutions and the fluorescence decreases.
Recently, three chlorosulfonylated tetradentate β-diketone-type ligands were synthesized (Figure 13.3) [18–20]. They differ from other β-diketones, because the fluorescence intensities of their Eu3+ complexes are not weakened on attaching a sulfonyl chloride group to the ligand. Compared with the bidentate β-diketone ligands, the tetradentate structures in these ligands increase the stabilities of the Eu3+complexes and also the fluorescence intensities because the ligands decrease the number of the coordinated water on the lanthanide ion and avoid the fluorescence quenching by water.
Among the three ligands, 4,4’-bis(1”,1”,1”,2”,2”,3”,3”-heptafluoro-4”,6”-hexanedion- 6”-yl)-chlorosulfo-o-terphenyl (BHHCT) is the most suitable label for time-resolved fluorometry. Compared with other two, BHHCT has the advantage that (i) it has only one sulfonyl chloride group, therefore cross labeling among several protein molecules does not occur, (ii) its Eu3+ complex maintains long fluorescence lifetime (400 to 700 µs) in various buffers, (iii) the relative fluorescence intensity (εφ) of the Eu3+ complex is considerably larger than those of other europium labels, and (iv) the Eu3+ complex (bound to bovine serum albumin (BSA)) has a relatively large stability constant (about 1010 M−1),
440 |
|
|
TAKUYA NISHIOKA, JINGLI YUAN, AND KAZUKO MATSUMOTO |
|||||||||||||
|
|
|
F2 |
|
|
|
|
|
|
|
|
|
|
|
||
F2C |
C |
|
|
|
|
|
|
|
|
|
SO2Cl |
|||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
||||||
|
O |
|
O |
|||||||||||||
|
|
|
|
O |
|
O |
|
|
|
|
|
|
|
|||
F2C |
|
|
|
|
|
|
|
|
SO2Cl |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
C |
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
F2 |
|
(BCDOT) |
|||||||||||
|
|
|
|
|
|
|
||||||||||
|
|
|
F2 |
|
|
|
|
|
|
S |
||||||
|
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
||
F C |
|
|
|
|
|
|
|
|
|
|
|
|
|
SO2Cl |
||
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
||||||
2 |
|
|
O |
O |
||||||||||||
|
|
|
||||||||||||||
|
|
|
O |
O |
S |
|||||||||||
|
|
|
|
|
||||||||||||
F2C |
|
|
|
|
|
|
|
|
|
|
|
|
|
SO2Cl |
||
C |
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
F2
(BCOT)
C3F7
O
O
ClO2S

O
O
(BHHCT) C3F7
FIGURE 13.3. Structures of three chlorosulfonylated tetradentate β-diketone labels. BCDOT = 1,10- bis(4”-chlorosulfo-1’,1”-diphenyl-4’-yl)-4,4,5,5,6,6,7,7-octafluorodecane-1,3,8,10-tetraone, BCOT = 1,10- bis(8’-chlorosulfodibenzothiophene-2’-yl)-4,4,5,5,6,6,7,7-octafluorodecane-1,3,8,10-tetraone, BHHCT = 4,4’- bis(1”,1”,1”,2”,2”,3”,3”-heptafluoro-4”,6”-hexanedion-6”-yl)chlorosulfo-o-terphenyl.
and so the complex is very stable in solutions of complex multi-component ones as used in bioassays. The ligand BHHCT is easy to conjugate to proteins through sulfonamide formation (protein-NH-SO2-label). The main drawback of BHHCT is its low solubility in water-based buffers, which makes it unsuitable for direct labeling of small molecules in aqueous solution. However, BHHCT-labeled BSA, the hapten-BSA conjugate, streptavidin (SA), antibodies, and other proteins and nucleic acids are soluble in water-based buffers.
Aromatic amine derivative-type ligands mostly consist of derivatives of pyridine, 2,2’- bipyridine, 2,2’,2”-terpyridine, and 1,10-phenanthroline [21–24]. Figure 13.4 shows the structures of four Eu3+ chelates with aromatic amine derived ligands, that can be covalently
FLUORESCENT LANTHANIDE LABELS WITH TIME-RESOLVED FLUOROMETRY |
441 |
|||||||||||||||||||||||||||
ClO2S |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
NH2 |
NH2 |
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
HN |
O HN |
O |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
Eu3+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
N |
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ClO2S |
|
|
|
|
|
|
|
|
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
N |
N Eu3+ |
N |
|
N |
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
(BCPDA-Eu3+) |
|
|
|
|
|
|
|
N |
N |
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(TBP-Eu3+) |
|
||||||||||
|
|
|
|
OCH3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
NH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO - |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
ClO2S |
|
|
|
|
|
|
|
|
|
|
2 |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Eu3+ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
N |
|
|
N |
N |
|
|
|
|
|
|
|
|
|
|
N |
|
|
N |
|
||||||||
N |
|
|
Eu3+ |
|
|
N |
|
ClO S |
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
CO |
- CO |
- |
|
|
|
|
|
|
CO - |
CO - |
2 |
|
|
|
|
|
|
|
|
|
|
|
- |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
2 |
2 |
|
|
|
|
|
2 |
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2 |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
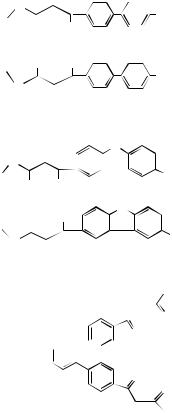
FIGURE 13.4. Structures of four fluorescent Eu3+ chelates with aromatic amine ligands that can be covalently bound to proteins.
bound to proteins, and among which 4,7-bis(chlorosulfophenyl)-1,10-phenanthroline-2,9- dicarboxylic acid (BCPDA)-Eu3+ and trisbipyridine cryptate (TBP)-Eu3+ are commercially used for europium fluorescence labels in TR-FIA.
13.3. TIME-RESOLVED FLUOROMETRY OF LANTHANIDE COMPLEXES
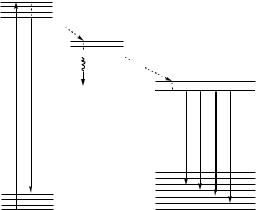
The main problem of the conventional fluorescence bioassay is the strong background signal including the fluorescence from the coexisting biological materials, the scattering light associated with Tyndall, Rayleigh, and Raman scattering, and the background luminescence from the optical components such as the cuvettes, filters and lenses. The elimination of the background signals is essential for highly sensitive detection. For this purpose, the time-resolved fluorometry using long-lived fluorescent lanthanide chelates is the most favorable method, since the background noise is short-lived with a lifetime of a nanosecond to a few microseconds, and easily removed by time-resolved measurement. The principle of the time-resolved fluorometric measurement is illustrated in Figure 13.5. After a sample is excited by a flash lamp, the fluorescence of all the molecules begins to decay exponentially. Since the short-lived background signal rapidly decreases, it can be effectively eliminated during the delay time. This permits to measure only the long-lived lanthanide fluorescence during the counting time with high sensitivity. Furthermore, the measurement is usually reiterated many times (usually one second per cuvette) to accumulate the signal and improve the signal to noise ratio.

442 |
TAKUYA NISHIOKA, JINGLI YUAN, AND KAZUKO MATSUMOTO |
 Cycle time (1000 s)
Cycle time (1000 s) 

Flash excitation
|
Short-lived background |
|
|
Fluorescence |
|
Long-lived lanthanide fluorescence |
|
Delay |
|
|
|
time |
|
|
|
|
|
|
|
|
|
Counting time |
|
|
|
Recovery time |
|
0 |
200 |
600 |
1000 |
Time ( s)
FIGURE 13.5. Principle of the time-resolved fluorometric measurement with a delay time of 200 µs, a counting time of 400 µs, and a cycle time of 1000 µs.
13.4. DNA HYBRIDIZATION ASSAY
DNA hybridization assay is one of the most widely used tools for diagnosis of infections, genetics, neoplasmic diseases, and microbial taxonomy [25–27]. A seven-color time-resolved fluorescence DNA hybridization assay was developed for the detection of amplified products of the polymerase chain reaction (PCR) of the seven human papilloma virus (HPV) types 16, 18, 31, 33, 35, 39, and 45, associated with cervix cancer [28]. In the method, seven combinations of the non-fluorescent lanthanide labels, Eu3+, Tb3+, Sm3+, Eu3+-Tb3+, Eu3+-Sm3+, Tb3+-Sm3+, and Eu3+-Tb3+-Sm3+ were used to label seven HPV type-specific oligonucleotide probes. After hybridization of the labeled probes with the immobilized target strains, the Eu3+ and Sm3+ fluorescences were measured after addition of the DELFIA fluorescence enhancement solution containing β-diketone, TOPO, and Triton X-100. Then, a solution containing 4-(2’,4’,6’-trimethoxyphenyl)pyridine-2,6- dicarboxylic acid and cetyltrimethylammonium bromide was added to make the Tb3+ complex fluorescent, and the Tb3+ fluorescence was measured. After the measurement, the PCR products from each of the seven different viral strains were correctly assigned by monitoring the contribution from each of the three lanthanide ions to the total fluorescence. Heinonen et al. developed a triple-label time-resolved fluorescence DNA hybridization assay method for detection of PCR amplification products of seven cystic fibrosis mutations in human blood [29]. In the method, 14 kinds of allele-specific oligonucleotides labeled with Eu3+, Sm3+, or Tb3+ complexes were used as wild-type specific and mutant-specific probes. After the biotinylated PCR products collected in the SA coated microtiter wells were denatured and hybridized with the 14 probes, the Eu3+, Sm3+, and Tb3+ fluorescences were measured with the same method as described above.
In addition, the enzyme amplified time-resolved fluorometric measurement system has been used for another DNA hybridization assay. Bortolin and co-workers reported
FLUORESCENT LANTHANIDE LABELS WITH TIME-RESOLVED FLUOROMETRY |
443 |
the quantitative measurement of DNA PCR products by using a digoxigenin-labeled PCR product (the specific capture probe was immobilized onto the microtiter well) or a digoxigenin-tailed specific probe (the PCR product was captured onto the microtiter well) for hybridization [30]. After the hybrids were reacted with ALP-labeled anti-digoxigenin antibody, 5’-fluorosalicylphosphate and Tb3+-EDTA were added for the coloration reaction and the time-resolved fluorescence measurement. Recently, this method was further improved for higher sensitivity by using a two-round enzyme amplification method [31]. In this improved method, digoxigenin-labeled target DNA was immobilized onto the antidigoxigenin antibody-coated microtiter wells and then hybridized with a biotinylated DNA probe. After the hybrid was reacted with horseradish peroxidase-labeled SA, a solution containing biotinylated tyramine and H2O2 was added to react with the horseradish peroxidase. This reaction results in the attachment of multiple biotin moieties to the solid phase. Then ALP-labeled SA was added to react with the immobilized biotin molecules. The coloration reaction and time-resolved fluorescence measurement were performed as described above. Although this method needs an additional step, it gives a 10-times enhanced signal-to-noise ratio compared with the previous method. The BHHCT-Eu3+-labeled SA-BSA was also employed for the DNA hybridization assay [32].
The procedures of the conventional DNA hybridization assay are tedious and timeconsuming, requiring immobilization of the target DNA on a solid support, prehybridization, hybridization, washing, and detection. In addition, the hybridization reaction in the solid-solution phase proceeds rather slow, which also makes the assay time-consuming. To circumvent this problem, the homogeneous DNA hybridization assay based on fluorescence resonance energy transfer (FRET) has been developed [33]. In this assay, two DNA probes, one labeled with biotin on the 3’-terminus and the other with Cy5 on the 5’-terminus were used. After hybridization, the BHHCT-Eu3+-labeled SA was added to react with biotin. When the target DNA is present, the BHHCT-Eu3+ and Cy5 come close to each other, and the energy transfer occurs from the Eu3+ complex to Cy5. Thus the target DNA can be detected by measuring the sensitized emission of Cy5 at 669 nm with the UV-light excitation and the normal fluorescence measurement mode or the time-resolved mode. This method overcomes the interference of the background emission, and gives a high sensitivity with the detection limit of 200 pM.
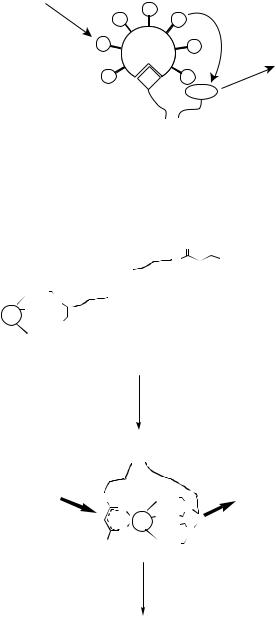
Another homogeneous DNA hybridization assay method has been developed, which is based on DNA-mediated formation of a ternary complex of EDTA-Eu3+-β-diketonate, to improve the detection limit [34]. The principle of the method is illustrated in Figure 13.7, in which two DNA probes are used; one labeled with the EDTA-Eu3+ chelate on the 5’-terminus and the other the β-diketone, 5-(4”-chlorosulfo-1’,1”-diphenyl-4’-yl)- 1,1,1,2,2-pentafluoro-3,5-pentanedione (CDPP), on the 3’-terminus. The two probes are complementary to the contiguous regions of the target DNA. After hybridization, two labels come close to each other to form the strongly fluorescent ternary complex of EDTA- Eu3+-β-diketonate, and thus the target DNA can be detected with the detection limit of 6 pM (0.6 fmol per assay) by time-resolved fluorescence measurement. Compared with the formation of the ternary complex in the absence of the target DNA, the formation of the complex between EDTA-Eu3+ and β-diketonate in the presence of the target DNA is strongly enhanced because the complex is fixed firmly in the contiguous region of the DNA template after the hybridization.
FLUORESCENT LANTHANIDE LABELS WITH TIME-RESOLVED FLUOROMETRY |
445 |
CONCLUSION
Time-resolved fluorometry by using lanthanide fluorescence labels is growing rapidly for applications in diagnostics and biotechnology. The combination of a lanthanide label and time-resolved fluorometric detection has been proved to efficiently remove undesired background fluorescence and detect even the very weak fluorescence which could not be detected with the conventional normal fluorometry using organic dyes. Such high detectability will innovate the performance of high through-put bio-chip technology and fluorescence microscopy. In DNA chip technique, amplification of DNA by PCR is unavoidable but should be kept as little as possible in order to avoid the risk of error in DNA duplication. In fluorescence microscopy, time-resolved measurement is expected to reduce the autofluorescence of the biomaterials and would give much better contrast. In this way, the lanthanide labels are expected to innovate the diagnostic and biotechnology world. Of course, the labels still need to be improved; they must have higher quantum yields, higher molar extinction coefficients, and longer fluorescence lifetimes, in order to have higher sensitivity and simpler analysis format. In addition, development of Eu3+, Sm3+, Tb3+, and Dy3+ four-color fluorescence labels is desired, since they could be applied in multiple-color time-resolved fluorescence imaging, four-color DNA sequencing, and DNA and protein microarrays. Currently Sm3+ and Dy3+ fluorescent complexes are only weakly fluorescent, and improvement of the fluorescence efficiency is highly desired. Synthesis of new fluorescent lanthanide complexes is therefore still an area that needs both more intensive and extensive effort of research.
REFERENCES
[1]E. Soini and I. Hemmil¨a. Clin. Chem., 25:353–361, 1979.
[2]I. Hemmil¨a. Clin. Chem., 31:359–370, 1985.
[3]E. Soini and T. Lovgren¨ . CRC Crit. Rev. Anal. Chem., 18:105–154, 1987.
[4]E.P. Diamandis. Clin. Biochem., 21:139–150, 1988.
[5]E.P. Diamandis and T.K. Christopoulos. Anal. Chem., 62:1149A–1157A, 1990.
[6]I. Hemmil¨a. Appl. Fluoresc. Technol., 1:1–8, 1988.
[7]I. Hemmil¨a. Scand. J. Clin. Lab. Invest., 48:389–400, 1988.
[8]E.F.G. Dickson, A. Pollak, and E.P. Diamandis. Pharmac. Ther., 66:207–235, 1995.
[9]I. Hemmil¨a and S. Webb. DDT, 2:373–381, 1997.
[10]J. Yuan and K. Matsumoto. Bunseki Kagaku, 48:1077–1083, 1999.
[11]K. Matsumoto and J. Yuan. Metal Ions in Biological Systems. A. Sigel and H. Sigel (eds.), Marcel Dekker, New York, Basel, Chapter 6, Vol. 40, pp.191–232, 2003.
[12]S.I. Weissman. J. Chem. Phys., 10:214–216, 1942.
[13]H.G. Huang, K. Hiraki, and Y. Nishikawa. Nippon Kagaku Kaishi, 66–70, 1981.
[14]R. Reisfeld and C.K. Jφrgensen. Lasers and Excited States of Rare Earths, Springer, Berlin, 1977.
[15]Y.-Y. Xu and I. Hemmil¨a. Anal. Chim. Acta, 256:9–16, 1992.
[16]W.D. Horrocks and D.R. Sudnick. J. Am. Chem. Soc., 101:334–340, 1979.
[17]A.G. Goryushko and N.K. Davidenko. Zh. Neorg. Khim., 25:2666–2668, 1980.
[18]J. Yuan and K. Matsumoto. Anal. Sci., 12:695–699, 1996.
[19]J. Yuan and K. Matsumoto. J. Pharm. Biomed. Anal., 15:1397–1403, 1997.
[20]J. Yuan, K. Matsumoto, and H. Kimura. Anal. Chem., 70:596–601, 1998.
[21]R.A. Evangelista, A. Pollak, B. Allore, E.F. Templeton, R.C. Morton, and E.P. Diamandis. Clin. Biochem., 21:173–177, 1988.
[22]G. Mathis. Clin Chem., 39:1953–1959, 1993.


 intersystem crossing
intersystem crossing



 energy transfer
energy transfer OOC
OOC
