
Micro-Nano Technology for Genomics and Proteomics BioMEMs - Ozkan
.pdf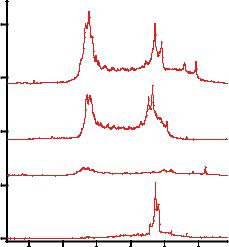
324 |
Z. HUGH FAN AND ANTONIO J. RICCO |
|
|
APG-IgG |
|
|
|
2.0 |
Complex |
|
APG |
(A.U.) |
|
|
||
|
|
|
|
|
Intensity |
1.5 |
|
|
250 pg/ L |
|
|
|
||
|
|
|
|
|
Fluorescence |
1.0 |
|
|
75 pg/ L |
|
|
|
||
0.5 |
|
|
25 pg/ L |
|
|
|
|
||
|
|
|
|
|
|
0.0 |
|
|
Control |
|
|
|
|
|
|
|
400 |
500 |
600 |
Time (s)
FIGURE 9.6. Detection of protein-protein interactions using CIEF in a microfabricated plastic device. Alexalabeled protein G (APG) and IgG were incubated at room temperature for 30 minutes. The mixture was diluted in 1:20 with ampholyte buffer before being subjected to miniaturized CIEF. The concentration of IgG was fixed at 250 pg/µL and that of APG is shown next to the electropherograms. The control sample has APG only. (Adapted from [64].)
whereas unlabeled IgG was not detectable. The IgG-APG complex was detectable as well. When the concentration of APG was increased from 25 pg/µL to 250 pg/µL while IgG was fixed at 250 pg/µL, the signal of the formed complex increased accordingly. The multiple peaks were explained by the heterogeneity of the APG (which may result from multiple-dye labeling). The results suggested APG was detectable at 25 pg/µL, corresponding to 50 fmol (5 × 10−14 mol) of protein when a sample of 40 µL was prepared.
9.5.2. Enzymatic Digestion for Protein Mapping
To determine protein identity and post-translational modifications, protein digestion with a proteolytic enzyme (e.g., trypsin) is typically carried out prior to mapping the masses of peptides. This enzymatic digestion preferably takes place on a solid support to eliminate the undesired autodigestion that would occur in solution. In addition, the solid support may function as a pre-concentration step (e.g., solid-phase extraction), which is very useful for those proteins present at very low concentrations.
A microfluidic device demonstrated recently contains both the functions of a solid-phase extractor (SPE) and an enzymatic microreactor [48]. The device was fabricated from a porous poly (butyl methacrylate-co-ethylene dimethacrylate) monolith prepared within a capillary. Photografting with irradiation through a mask was then used to selectively functionalize a portion of the monolith, introducing reactive poly (2-vinyl-4,4-dimethylazlactone) chains to enable the subsequent attachment of trypsin, thereby creating an enzymatic microreactor with high proteolytic activity. The remaining
PLASTIC MICROFLUIDIC DEVICES FOR DNA AND PROTEIN ANALYSES |
325 |
|||||
|
|
|
|
|
|
|
|
20 mm |
|
5 mm |
|
||
|
|
|
|
|
|
|
|
Microreactor |
|
||||
|
|
SPE |
|
|||
|
|
|
|
|
|
|
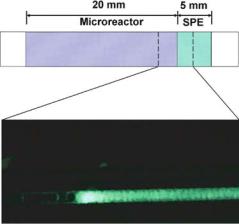
FIGURE 9.7. Scheme and fluorescence microscope image of the monolithic dual-function device used for the digestion of labeled casein and capture of fluorescent peptides. When 5 µL of 10 µg/mL BODIPY-labeled casein solution in 50 mM Tris buffer (pH 8.0) is pumped at a flow rate of 0.5 µL/min through the enzyme reactor on the left, digestion takes place, producing peptides. The resulting peptides are collected in the hydrophobic SPE on the right. After washing the monolith with 10 µL of Tris buffer to remove all unbound species, the enzyme reactor (left) exhibits no fluorescence while the SPE (right) shows strong fluorescence, indicating successful digestion and extraction. (Adapted from [48]; published with permission.)
unmodified hydrophobic monolith served as a micro SPE. Figure 9.7 shows schematically the portion of the device containing a SPE and a microreactor with immobilized enzyme.
The dual function of the device was demonstrated using BODIPY-labeled casein. Casein consists of intra-molecularly quenched fluorescing moieties that are unquenched only by proteolytic digestion. When a casein solution was pumped through the enzyme reactor on the left in Figure 9.7, digestion occurred, producing peptides. The resulting peptides were collected in the hydrophobic SPE on the right. After washing the monolith with a buffer solution to remove all unbound species, the left part of the monolith containing immobilized trypsin exhibited no fluorescence while the right portion containing SPE with extracted, fluorescently labeled peptides showed strong fluorescence. The presence of captured fluorescent species confirmed the digestive function of the device and the high fluorescence of peptides retained in the hydrophobic part of the monolith. The dual function of the device was further demonstrated by concentrating myoglobin, followed by elution and digestion, prior to coupling with MS for peptide mass mapping [48].
In addition, several research groups reported using microfluidic devices to immobilize enzymes for a variety of other applications. For instance, Crooks’ research group developed a method for determining enzyme kinetics using a continuous-flow microfluidic system [59]. The method involves immobilizing the enzyme on microbeads, then packing the microbeads into a chip-based microreactor. When the substrate flows over the packed bed, the signal is collected and data are analyzed in a way similar to conventional measurements based on the Michaelis-Menten equation. They studied the horseradish peroxidase-catalyzed reaction between hydrogen peroxide and N -acetyl-3,7-dihydroxyphenoxazine to yield fluorescent resorufin. The experimental results indicated the microfluidics-based method yielded the
326 |
Z. HUGH FAN AND ANTONIO J. RICCO |
same result as conventional methods. The advantages of the continuous-flow microfluidic system include rapid determination of enzyme kinetics and less consumption of reagents and enzymes.
CONCLUDING REMARKS
The examples in this chapter exemplify the sort of DNA and protein analyses that can be accomplished in plastic microfluidic devices. Sample preparation, including PCR, purification of DNA fragments, and enzymatic digestion of proteins have been demonstrated in custom-designed devices; some of them have been integrated with separations. In addition, plastic microfluidic devices were illustrated to be capable of performing DNA sequencing based on electrophoretic separation, and isoelectric focusing was shown as a means to study protein-protein interactions. To realize the “lab-on-a-chip” vision articulated by Manz and Harrison in the early 1990’s [20, 40], further integration of these devices with various hardware and accessories must be achieved, in addition to addressing the challenges associated with macro-micro interfaces.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge contributions from our former coworkers at ACLARA BioSciences, Inc., particularly our co-authors on the several ACLARA papers cited here. We thank Professors Soper and Frechet for providing Figures 9.5 and 9.7, respectively. ZHF acknowledges the financial support from McKnight Brain Institute, the startup fund from the University of Florida, and the grants from National Aeronautics and Space Administration (NASA) via UF Space Biotechnology and Commercial Applications Program, UCF-UF Space Research Initiative, and Hydrogen Research for Spaceport and Space Based Applications.
REFERENCES
[1]R.C. Anderson, G.J. Bogdan, Z. Barniv, T.D. Dawes, J. Winkler, and K. Roy. Proc. 1997 International Conf. Solid-State Sensors and Actuators, p. 477–480, 1997.
[2]C. Backhouse, M. Caamano, F. Oaks, E. Nordman, A. Carrillo, B. Johnson, and S. Bay. Electrophoresis, 21:50–156, 2000.
[3]D.R. Baker. Capillary Electrophoresis, Chapter 2, John Wiley & Sons Inc., New York, 1995.
[4]S.L. Barker, M.L. Tarlov, H. Canavan, J.J. Hickman, and L.E. Locascio. Anal. Chem. 72:4899–4903, 2000.
[5]G. Binyamin, T.D. Boone, H.S. Lackritz, A.J. Ricco, A.P. Sassi, and S.J. Williams. Plastic microfluidic devices: electrokinetic manipulations, life science applications, and production technologies, In R.E. Oosterbroek and A. van den Berg (eds.) “Lab-on-a-Chip: Miniaturized Systems for (Bio)Chemical Analysis and Synthesis, ”’ Elsevier, Amsterdam, p. 83–112, 2003.
[6]T.D. Boone, H.H. Hooper, and D.S. Soane. Technical Digest of the 1998 Solid-State Sensor and Actuator Workshop, Transducers Research Foundation, Cleveland, p. 87–92, 1998.
[7]T.D. Boone, Z.H. Fan, H.H. Hooper, A.J. Ricco, H. Tan, and S.J. Williams. Anal. Chem. 74:78A–86A, 2002.
[8]M.A. Burns, B.N. Johnson, S.N. Brahmasandra, K. Handique, J.R. Webster, M. Krishnan, T.S. Sammarco, P.M. Man, D. Jones, D. Heldsinger, C.H. Mastrangelo, and D.T. Burke. Science, 282:484–487, 1998.
PLASTIC MICROFLUIDIC DEVICES FOR DNA AND PROTEIN ANALYSES |
327 |
[9]J. Chan, A.T. Timperman, T. Qin, and R. Aebersold. Anal. Chem., 71:4437–4444, 1999.
[10]N. Chiem and D.J. Harrison. Anal. Chem., 69:373–378, 1997.
[11]C.S. Effenhauser, G.J.M. Bruin, A. Paulus, and M. Ehrat. Anal. Chem. 69:3451–3457, 1997.
[12]A.G. Ewing, R.A. Wallingford, and T.M. Olefirowicz. Anal. Chem., 61:292A–303A. 1989.
[13]Z.H Fan and D.J. Harrison. Anal. Chem., 66:177–184, 1994
[14]Z.H. Fan, S. Mangru, R. Granzow, Ho, W Heaney, Q. Dong, and R. Kumar, Anal. Chem., 71:4851–4859, 1999.
[15]Z.H. Fan, W. Tan, H. Tan, X.C. Qiu, T.D. Boone, P. Kao, A.J. Ricco, M. Desmond, S. Bay, and K. Hennessy.
Plastic Microfluidic Devices for DNA Sequencing and Protein Separations, Micro Total Analysis Systems 2001, Kluwer Academic Publishers, Boston, pp. 19–21, 2001.
[16]G.S. Fiorini, G.D.M. Jeffries, D.S.W. Lim, C.L. Kuyper, and D.T. Chiu. Lab on a chip, 3:158–163, 2003.
[17]P.R. Gascoyne and J. Vykoukal. Electrophoresis, 23:1973–83, 2002.
[18]B.B. Haab and R.A. Mathies. Anal. Chem., 71:5137–5145, 1999.
[19]A.G. Hadd, D.E. Raymond, J.W. Halliwell, S.C. Jacobson, and J.M. Ramsey. Anal. Chem., 69:3407–3412, 1997.
[20]D.J. Harrison, A. Manz, Z. Fan, H. Ludi,¨ and H.M. Widmer. Anal. Chem., 64:1926–1932, 1992.
[21]D.J. Harrison, P.G. Glavina, and A. Manz. Sensors and Actuators B, 10:107–116, 1993.
[22]A.E. Herr, J.I. Molho, J.G. Santiago, T.W. Kenny, D.A. Borkholder, G.J. Kintz, P. Belgrader, and M.A. Northrup. Electrophoresis, 22:2291–2295, 2001.
[23]A.E. Herr, J.I. Molho, K.A. Drouvalakis, J.C. Mikkelsen, P.J. Utz, J.G. Santiago, and T.W. Kenny. Anal. Chem., 75:1180–1187, 2003.
[24]O. Hofmann, D. Che, K.A. Cruickshank, and U.R. Muller. Anal. Chem., 71:678–686, 1999.
[25]S.C. Jacobson, R. Hergenroder, L.B. Koutny, R.J. Warmack, and J.M. Ramsey. Anal. Chem., 66:1107–1113, 1994.
[26]J. Kameoka, H.G Craighead, H. Zhang, and J. Henion. Anal. Chem., 73:1935–1941, 2001.
[27]C.G. Koh, W. Tan, M. Zhao, A.J. Ricco, and Z.H. Fan. Anal. Chem., 75:4591–4598, 2003.
[28]M.U. Kopp, A.J. de Mello, and A. Manz. Science, 280:1046–1048, 1998.
[29]P.C.H Li and D.J. Harrison. Anal. Chem., 69:1564–1568, 1997.
[30]Z. Liang, N. Chiem, G. Ocvirk, T. Tang, K. Fluri, and D.J. Harrison. Anal. Chem., 68:1040 –1046, 1996.
[31]A.J. Link, J. Eng, D.M. Schieltz, E. Carmack, G.J. Mize, D.R. Morris, B.M. Garvik, and J.R. III Yates. Nat. BioTechnol., 17:676–682, 1999.
[32]Y. Liu, C.B. Rauch, R.L. Stevens, R. Lenigk, J. Yang, D.B. Rhine, and P. Grodzinski. Anal. Chem., 74:3063– 3070, 2002.
[33]S. Liu, H. Ren, Q. Gao, D.J. Roach, R.T. Loder, T.M. Armstrong, Q. Mao, L. Blaga, D.L. Barker, and S.B. Jovanovich. Proc. Natl. Acad. Sci. USA, 97:5369–5374, 2000.
[34]S. Liu, Y. Shi, W.W. Ja, and R.A. Mathies. Anal. Chem., 71:566–573, 1999.
[35]K.D. Lukacs and J.W. Jorgenson. J. High Res. Chromatogr. & Chromatogr. Commun., 8:407–411, 1985.
[36]G. MacBeath and S.L. Schreiber. Science, 289:1760–1763, 2000.
[37]S.E. McBride, R.M. Moroney, and W. Chiang. ‘Electrohydrodynamic pumps for high-density microfluidic arrays. In D.J. Harrison and A van den Berg (eds.), Micro Total Analysis System’, Kluwer Academic Publishers, pp. 45–48, 1998.
[38]K. Macounova, C.R. Cabrera, M.R. Holl, and P. Yager. Anal. Chem., 72:3745–3751, 2000.
[39]R.S. Madabhushi. Electrophoresis, 19:224–230, 1998.
[40]A. Manz, J.C. Fettinger, E. Verpoorte, H. Lude, H. M. Widmer, and D.J. Harrison. Trends in Anal. Chem., 10:144–149, 1991.
[41]A. Manz, Y. Miyahara, J. Miura, Y. Watanabe, H. Miyagi, and K. Sato. Sensors and Actuators, B1:249–255, 1990.
[42]R.M. McCormick, R.J. Nelson, M.G. Alonso-Amigo, D.J. Benvegnu, and H.H. Hooper. Anal. Chem., 69:2626–2630, 1997.
[43]J.C. McDonald, D.C. Duffy, J.A. Anderson, D.T. Chiu, H. Wu, O.J. Schueller, and G.M. Whitesides. Electrophoresis, 21:27–40, 2000.
[44]M.A. Northrup, B. Benett, D. Hadley, P. Landre, S. Lehew, J. Richards, and P. Stratton. Anal. Chem., 70:918– 922, 1998.
[45]M.A. Northrup, K.F. Jensen, and D.J. Harrison. Micro Total Analysis System’2003, The Transducer Research Foundation, 2003.
328 |
Z. HUGH FAN AND ANTONIO J. RICCO |
[46]R.E. Oosterbroek and A. van den Berg. ‘Lab-on-a-Chip: Miniaturized Systems for (Bio)Chemical Analysis and Synthesis’, Elsevier, Amsterdam, 2003.
[47]S. Park and R. Raines. Nature Biotech., 18:847–851, 2000.
[48]D.S. Peterson, T. Rohr, F. Svec, and J.M.J. Frechet. Anal. Chem. 75:5328–5335, 2003.
[49]D.L. Pugmire, E.A. Waddell, R. Haasch, M.J. Tarlov, and L.E. Locascio. Anal. Chem., 74:871–878, 2002.
[50]R. Riffon, K. Sayasith, H. Khalil, P. Dubreuil, M. Drolet, and J.J. Lagace. Clin. Microbiol., 39:2584–2589, 2001.
[51]M.A. Roberts, J.S. Rossier, P. Bercier, and H.H. Girault. Anal. Chem. 69:2035–2042, 1997.
[52]R. Rodriguez-Diaz, T. Wehr, and M. Zhu. Electrophoresis, 18:2134–2144, 1997.
[53]M.G. Roper, J.G. Shackman, G.M. Dahlgren, and R.T. Kennedy. Anal. Chem. 75:4711–4717, 2003.
[54]J.S. Rossier, M.A. Roberts, R. Ferrigno, and H.H. Girault. Anal. Chem., 71:4294–4299, 1999.
[55]E. Sahlin, A.T. Beisler, S.J. Woltman, and S.G. Weber. Anal. Chem., 74:4566–4569, 2002.
[56]A.P. Sassi, Q. Xue, and H.H. Hooper. Amer. Lab. Oct.:36–41, 2000.
[57]K. Sato, M. Yamanaka, H. Takahashi, M. Tokeshi, H. Kimura, and T. Kitamori. Electrophoresis, 23:734–739, 2002.
[58]D. Schmalzing, A. Adourian, L. Koutny, L. Ziaugra, P. Matsudaira, and D. Ehrlich. Anal. Chem., 70:2303– 2310, 1998.
[59]G.H. Seong, J. Heo, and R.M. Crooks. Anal. Chem., 75(13):3161–3167, 2003.
[60]Y. Shen, S.J. Berger, and R.D. Smith. Anal. Chem., 72:4603–4607, 2000.
[61]S.A. Soper, S.M. Ford, S. Qi, R.L. McCarley, K. Kelly, and M.C. Murphy. Anal. Chem., 72:643A–651A, 2000.
[62]S.A. Soper, S.M. Ford, Y. Xu, S. Qi, S. McWhorter, S. Lassiter, D. Patterson, and R.C.J. Bruch. Chromatogr. A., 853:107–120, 1999.
[63]E. Tamaki, K. Sato, M. Tokeshi, K. Sato, M. Aihara, and T. Kitamori. Anal. Chem., 74:1560–1564, 2002.
[64]W. Tan, Z.H. Fan, C.X. Qiu, A.J. Ricco, and I. Gibbons. Electrophoresis, 23:3638–3645, 2002.
[65]Q. Tang, A.K. Harrata, and C.S. Lee. Anal. Chem., 69:3177–3182, 1997
[66]S.C. Terry, J.H. Jerman, and J.B. Angell. IEEE Trans. Electron. Devices, ED-26:1880–1886, 1979.
[67]T. Thorsen, S.J. Maerkl, and S.R. Quake. Science, 298:580–584, 2002.
[68]J. Wang and M. Pumera. Anal Chem., 74:5919–23, 2002.
[69]Y. Wang, B. Vaidya, H.D. Farquar, W. Stryjewski, R.P. Hammer, R.L. McCarley, S.A. Soper, Y.W. Cheng, and F. Barany. Anal. Chem., 75:1130–40, 2003.
[70]L.C. Waters, S.C. Jacobson, N. Kroutchinina, J. Khandurina, R.S. Foote, and J.M. Ramsey. Anal. Chem., 70:5172–5176, 1998.
[71]J. Wen, Y. Lin, F. Xiang, D.W. Matson, H.R. Udseth, and R.D. Smith, 21:191–197, 2000.
[72]P. Wilding, M.A. Shoffner, and L. Kricka. J. Clin. Chem., 40:1815–1818, 1994.
[73]C.T. Wittwer, D.J. Garling. Biotechniques, 10:76–83, 1991.
[74]A.T. Woolley, D. Hadley, P. Landre, A.J. deMello, R.A. Mathies, and M.A. Northrup. Anal. Chem., 23:4081– 4086, 1996.
[75]A.T. Woolley and R.A. Mathies. Anal. Chem., 67:3676–3680, 1995.
[76]A.T. Woolley and R.A. Mathies. Proc. Natl. Acad. Sci. USA, 91:11348–11352, 1994.
[77]Y. Xu, B. Vaidya, A.B. Patel, S.M. Ford, R.L. McCarley, and S.A. Soper, Anal. Chem. 75:2975–2984, 2003.
[78]Q. Xue, A. Wainright, S. Ganakhedkar, and I. Gibbons. Electrophoresis, 18:4000–4007, 2001.
[79]X. Zuo and D.W Speicher. Proteomics, 2:58–68, 2002.
10
Centrifuge Based Fluidic
Platforms
Jim V. Zoval1 and M.J. Madou2
1University of California, Irvine Department of Mechanical and Aerospace Engineering
2University of California, Irvine Department of Mechanical and Aerospace Engineering and Department of Biomedical Engineering
10.1. INTRODUCTION
Once it became apparent that individual chemical or biological sensors used in complex samples would not attain the hoped for sensitivity or selectivity, wide commercial use became severely hampered and sensor arrays and sensor instrumentation were proposed instead. It was projected that by using orthogonal sensor array elements (e.g., in electronic noses and tongues) selectivity would be improved dramatically [1]. Instrumentation—it was envisioned—would reduce matrix complexities through filtration, separation, and concentration of the target compound, while, at the same time, ameliorating selectivity and sensitivity of the overall system by frequent recalibration and washing of the sensors. Through miniaturization of analytical equipment (using microfluidics), shortcomings associated with large and expensive instrumentation may potentially be overcome: reduction in reagent volumes, favorable scaling properties of several important instrument processes (basic theory of hydrodynamics and diffusion predicts faster heating and cooling and more efficient chromatographic and electrophoretic separations in miniaturized equipment) and batch-fabrication which may enable low cost, disposable instruments to be used once and then thrown away to prevent sample contamination [2]. Micromachining (MEMS) might also allow co-fabrication of many integrated functional instrument blocks. Tasks that are now performed in a series of conventional bench top instruments could then be combined into one unit, reducing labor and minimizing the risk of sample contamination.
330 |
JIM V. ZOVAL AND M.J. MADOU |
Today it appears that sensor array development in electronic noses and tongues has slowed down because of the lack of highly stable chemical and biological sensors: too frequent recalibration of the sensors and relearning of the pattern recognition software is putting a damper on the original enthusiasm for this sensor approach. In the case of miniaturization of instrumentation through the application of microfluidics, progress was made in the development of platforms for high-throughput screening (HTS) as evidenced by new products introduced, by, for example, Caliper and Tecan Boston [3, 4]. In contrast, for sensing and diagnostic applications, not much progress was made using miniaturized analytical equipment. There have been platforms developed for a limited amount of human and veterinary diagnostic test that do not require complex fluidic design, for example, Abaxis [5]. In this review paper we are, in a narrow sense, summarizing the state of the art of compact disc (CD) based microfluidics and in a broader sense we are comparing the technical barriers involved in applying microfluidics to sensing and diagnostics as opposed to applying such techniques to high throughput screening (HTS). It will quickly become apparent that the former poses the more severe technical challenges and as a result the promise of lab-on-a-chip has not been fulfilled yet.
10.2. WHY CENTRIFUGE AS FLUID PROPULSION FORCE?
There are various technologies for moving small quantities of fluids or suspended particles from reservoirs to mixing and reaction sites, to detectors, and eventually to waste or to a next instrument. Methods to accomplish this include syringe and peristaltic pumps, electrochemical bubble generation, acoustics, magnetics, DC and AC electrokinetics, centrifuge, etc. In Table 10.1 we compare four of the more important and promising fluid propulsion means [6]. The pressure that mechanical pumps have to generate to propel fluids through capillaries is higher the narrower the conduit. Pressure and centrifugal force are both volume-dependent forces, which scale as L3(in this case L is the characteristic length corresponding to the capillary diameter). Piezoelectric, electroosmotic, electrowetting and electrohydrodynamic (EHD) pumping (the latter two are not shown in Table 10.1) all scale as surface forces (L2), which represent more favorable scaling behavior in the micro-domain (propulsion forces scaling with a lower power of the critical dimension become more attractive in the micro-domain) and lend themselves better to pumping in smaller and longer channels. In principle, this should make pressureand centrifuge-based systems less favorable but other factors turn out to be more decisive; despite better scaling of the non-mechanical pumping approaches in Table 10.1, almost all biotechnology equipment today remain based on traditional external syringe or peristaltic pumps. The advantages of this approach are that it relies on well-developed, commercially available components and that a very wide range of flow rates is attainable. Although integrated micromachined pumps based on two one-way valves may achieve a precise flow control on the order of 1 µl/min with fast response, high sensitivity, and negligible dead volume, these pumps generate only modest flow rates and low pressures, and consume a large amount of chip area and considerable power.
Acoustic streaming is a constant (DC) fluid motion induced by an oscillating sound field at a solid/fluid boundary. A disposable fluidic manifold with capillary flow channels can simply be laid on top of the acoustic pump network in the reader instrument. The method

CENTRIFUGE BASED FLUIDIC PLATFORMS |
331 |
TABLE 10.1. Comparison of microfluidics propulsion techniques
|
|
Fluid Propulsion Mechanism |
|
|
|
|
|
|
|
Comparison |
Centrifuge |
Pressure |
Acoustic |
Electrokinetic |
|
|
|
|
|
Valving solved ? |
Yes for liquids no |
Yes for liquids and |
No solution shown |
Yes for liquids, no |
|
for vapor |
vapor |
yet for liquid or |
for vapor |
|
|
|
vapor |
|
Maturity |
R&D stage |
Products available |
Research |
Products available |
Propulsion force |
Density and |
Generic |
Generic |
pH, ionic strength |
influenced by |
viscosity |
|
|
|
Power source |
Rotary motor |
Pump, Mechanical |
5 to 40 V |
10 kV |
|
|
roller |
|
|
Materials |
Plastics |
Plastics |
Piezoelectrics |
Glass, plastics |
Scaling |
L3 |
L3 |
L2 |
L2 |
Flow rate |
From less than |
Very wide range |
20 µl/sec |
0.001–1 µl/sec |
|
1nL/sec to |
(less than nL/sec |
|
|
|
greater than |
to L/sec) |
|
|
|
100 µl/sec |
|
|
|
General remarks |
Inexpensive CD |
Standard technique. |
Least mature of |
Mixing difficult. |
|
drive, mixing is |
Difficult to |
the four |
High voltage |
|
easy, most |
miniaturize and |
techniques. |
source is |
|
samples possible |
multiplex. |
Might be too |
dangerous and |
|
(including |
|
expensive. |
many parameters |
|
cells). Better for |
|
Better for |
influence |
|
diagnostics. |
|
smallest |
propulsion, better |
|
|
|
samples. |
for smallest |
|
|
|
|
samples (HTS) |
|
|
|
|
|
is considerably more complex to implement than electro-osmosis (described next) but the insensitivity of acoustic streaming to the chemical nature of the fluids inside the fluidic channels and its ability to mix fluids make it a potentially viable approach. A typical flow rate measured for water in a small metal pipe lying on a piezoelectric plate is 0.02 cc/s at 40 V, peak to peak [7]. Today acoustic streaming as a propulsion mechanism remains in the research stage.
Electro-osmotic pumping (DC electrokinetics) in a capillary does not involve any moving parts and is easily implemented. All that is needed is a metal electrode in some type of a reservoir at each end of a small flow channel. Typical electro-osmotic flow velocities are on the order of 1 mm/sec with a 1200 V/cm applied electric field. For example, in free-flow capillary electrophoresis work by Jorgenson, electro-osmotic flow of 1.7 mm/sec was reported [8]. This is fast enough for most analytical purposes. Harrison et al. achieved electroosmotic pumping with flow rates up to 1 cm/sec in 20 µm capillaries that were micromachined in glass [9]. They also demonstrated the injection, mixing and reaction of fluids in a manifold of micromachined flow channels without the use of valves. The key aspect for tight valving of liquids at intersecting capillaries in such a manifold is the suppression of convective and diffusion effects. The authors demonstrated that these effects can be controlled by the appropriate application of voltages to the intersecting channels simultaneously. Some disadvantages of electro-osmosis are the required high voltage (1–30 kV power supply) and direct electrical-to-fluid contact with resulting sensitivity of
332 |
JIM V. ZOVAL AND M.J. MADOU |
LabCDTM Reader
LabCDTM Disc
Analysis Optics |
Spectrophotomeric |
|
|
read cuvette |
Fluidics manifold |
||
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Drive motor |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
||||
|
Cuvette |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Informatics CD optics
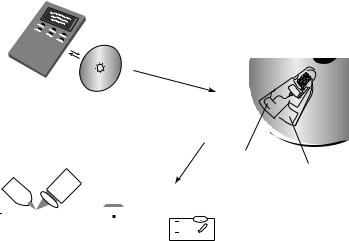
FIGURE 10.1. LabCDTM Instrument and disposable disc. Here, the analytical result is obtained through reflection spectrophotometry.
flow rate to the charge of the capillary wall and to the ionic strength and pH of the solution. It is consequently more difficult to make it into a generic propulsion method. For example, liquids with high ionic strength cause excessive Joule heating; it is therefore difficult or impossible to pump biological fluids such as blood and urine.
Using a rotating disc, centrifugal pumping provides flow rates ranging from less than 10 nL/s to greater than 100 µL/s depending on disc geometry, rotational rate (RPM), and fluid properties (see Figure 10.1)[10]. Pumping is relatively insensitive to physicochemical properties such as pH, ionic strength, or chemical composition (in contrast to AC and DC electrokinetic means of pumping). Aqueous solutions, solvents (e.g., DMSO), surfactants, and biological fluids (blood, milk, and urine) have all been pumped successfully. Fluid gating, as we will describe in more detail further below, is accomplished using “capillary” valves in which capillary forces pin fluids at an enlargement in a channel until rotationally induced pressure is sufficient to overcome the capillary pressure (at the socalled burst frequency) or by hydrophobic methods. Since the types and the amounts of fluids one can pump on a centrifugal platform spans a greater dynamic range than for electrokinetic and acoustic pumps, this approach seems more amenable to sample preparation tasks than electrokinetic and acoustic approaches. Moreover miniaturization and multiplexing are quite easily implemented. A whole range of fluidic functions including valving, decanting, calibration, mixing, metering, sample splitting, and separation can be implemented on this platform and analytical measurements may be electrochemical, fluorescent or absorption based and informatics embedded on the same disc could provide test-specific information.
A most important deciding factor in choosing a fluidic systems is the ease of implementing valves; the method that most elegantly solves the valving issue is already commercially

CENTRIFUGE BASED FLUIDIC PLATFORMS |
333 |
accepted, even if the scaling is not the most favorable namely in the use of traditional pumps. In traditional pumps two one-way valves form a barrier for both liquids and vapors. In the case of the micro-centrifuge, valving is accomplished by varying rotation speed and capillary diameter. Thus, there is no real physical valve required for stopping water flow, but as in the case of acoustic and electrokinetic pumping there is no simple means to stop vapors from spreading over the whole fluidic platform. If liquids need to be stored for a long time on the disposable, as often is the case for use in sensing and diagnostics, valves must be barriers for both liquid and vapor. Some timid attempts at implementing vapor barriers on the CD will be reported in this review.
From the preceding comparison of fluidic propulsion methods for sensing and diagnostic applications, centrifugation in fluidic channels and reservoirs crafted in a CD-like plastic substrate as shown in Figure 10.1 constitutes an attractive fluidic platform.
10.3. COMPACT DISC OR MICRO-CENTRIFUGE FLUIDICS
10.3.1. How it Works
CD fluid propulsion is achieved through centrifugally induced pressure and depends on rotation rate, geometry and location of channels and reservoirs, and fluid properties. Madou et al [11] and Duffy et al [9] characterized the flow rate of aqueous solutions in fluidic CD structures and compared the results to simple centrifuge theory. The average velocity of the liquid (U) from centrifugal theory is given as:
U = D2h ρω2 |
|
r/32µL , |
(10.1) |
r |
and the volumetric flow rate (Q) as:
Q = UA |
(10.2) |
where Dh is the hydraulic diameter of the channel (defined as 4A/P, where A is the crosssectional area and P is the wetted perimeter of the channel), ρ is the density of the liquid, ω is the angular velocity of the CD, r is the average distance of the liquid in the channels to the center of the disc, r is the radial extent of the fluid, µ is the viscosity of the solution, and L is the length of the liquid in the capillary channel (see also Figure 10.2A). Flow rates, ranging from 5nL/s to > 0.1mL/s, have been achieved by various combinations of rotational speeds (from 400 to 1600rpm), channel widths (20–500µm), and channel depths (16–340µm). The experimental flow rates were compared to rates predicted by the theoretical model and exhibited an 18.5% coefficient of variation. The authors note that experimental errors in measuring the highest and lowest flow rates made for the largest contribution to this coefficient of variation. The absence of systematic deviation from theory validates the model for describing flow in microfluidic channels under centripetal force. Duffy et al [10] measured flow rates of water, plasma, bovine blood, three concentrations of hematocrit, urine, dimethyl sulfoxide (DMSO), and PCR products and report that centrifugal pumping is relatively insensitive to such physiochemical properties as ionic strength, pH, conductivity,
