
- •Adrenaline (again)
- •Adrenergic receptor agonists and antagonists
- •Acetylcholine receptors
- •Acetylcholine
- •Cholinergic receptor subtypes
- •Nicotinic receptors
- •Muscarinic receptors
- •Nicotinic receptors are ion channels
- •Architecture of the nicotinic receptor
- •Other ligand-gated ion channels
- •The 7TM superfamily of G-protein-linked receptors
- •Categories of 7TM receptor
- •Receptor diversity: variation and specialization
- •Binding of low-molecular-mass ligands
- •Calcium sensors and metabotropic receptors
- •Proteinase-activated receptors (PARs)
- •The adhesion receptor subfamily
- •Frizzled
- •Receptor–ligand interaction and receptor activation
- •A two-state equilibrium description of receptor activation
- •Receptor dimerization
- •Transmitting signals into cells
- •The receptor and the effector: one and the same or separate entities?
- •Mixing and matching receptors and effectors
- •Intracellular 7TM receptor domains and signal transmission
- •Adrenaline (yet again)
- •References
Signal Transduction
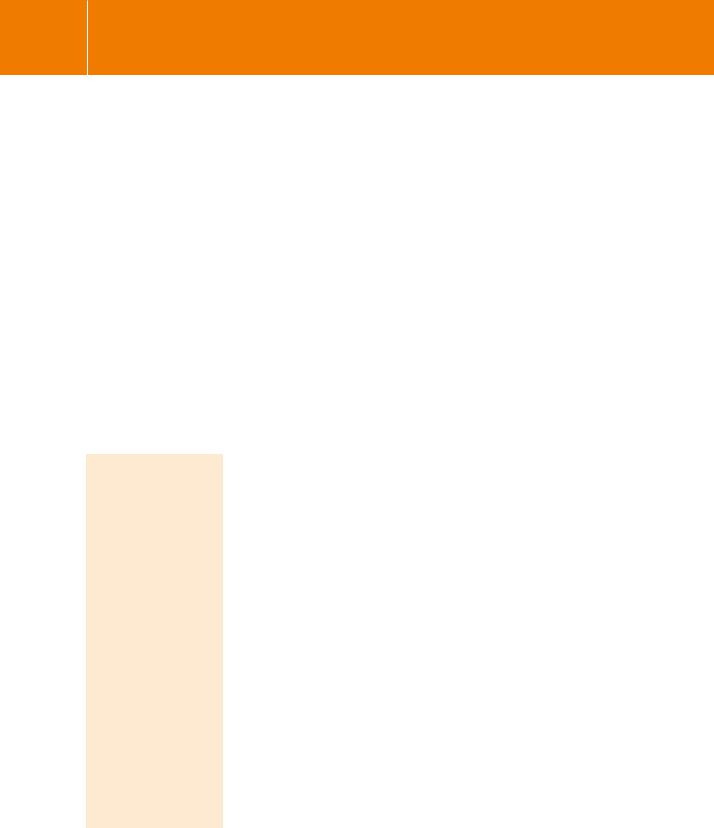
•Nicotine: named after Jacques Nicot, the French ambassador to Lisbon who introduced the tobacco plant
Nicotiana tabacum into France in 1560.
•Muscarine: a product of the poisonous mushroom, Amanita muscaria.
•Atropine: from the Greek a-tropos, meaning no turning or inflexible, referring to the condition of the pupil when dilated by
treatment with atropine. It is an alkaloid extracted from the berries of deadly nightshade,
Atropa belladonna. The content of a single berry is sufficient to kill an adult human.
Clermont even went so far as to comment on its taste and one may wonder that he ever survived to tell the tale. Further organophosphorus compounds were developed with the aim of preparing nasty surprises for flies (parathion, the most widely used insecticide of this class) and subsequently even nastier surprises for soldiers (and, sadly, civilians too). These nerve gases, now regarded as weapons of mass destruction (wmds) include sarin (isopropylmethylphosphonofluoridate), originally developed in Nazi Germany. Four years after the attack by Saddam Hussein’s forces on the city of Halabja in March 1988, an estimated 5000 people were killed and subsequent soil samples revealed traces of sarin breakdown products. The LD50 for humans is reckoned to be (not tested!) of the order of 10 g kg 1. When sarin was released on the Tokyo metro system on 20 March 1995 by zealots of the Aum cult, 12 people were killed and 5500 were injured.
All these compounds take their effects by alkylphosphorylation of active site serines, not only of acetylcholinesterase, but also of the serine esterases and serine proteinases (such as trypsin, thrombin, etc.). Clearly, the rapid removal of acetylcholine from the synapse is as essential for the process
of neurotransmission as is its release. Interestingly however, some of the ‘reversible’ cholinesterase inhibitors, such as neostigmine, are used in the treatment of glaucoma, and myasthenia gravis, with the aim of restoring the availability of acetylcholine.
With the threat of military and terrorist abuse of these noxious agents attention has turned to the matter of antidotes. Currently atropine is administered to prevent over-occupation of the acetylcholine receptors. In addition, casualties are treated with nucleophilic agents based on 2,3-butanedione monoxime with the aim of dephosphorylating and so reactivating the poisoned acetylcholine esterase.6,7
Cholinergic receptor subtypes
Acetylcholine receptors fall into two classes, originally distinguished by their responses to the pharmacological agonists, nicotine and muscarine. In this section we also give brief consideration to related receptors that respond to amino acid neurotransmitters.
Nicotinic receptors
At the neuromuscular junction acetylcholine acts through nicotinic cholinergic receptors to stimulate the contraction of skeletal muscle. It also transmits signals in the ganglia of the autonomic nervous system. The receptors are cation channels which conduct both Na and K , the principal cations present in cells and extracellular fluid. Nicotinic receptors
are antagonized by tubocurarine. Neither nicotine nor tubocurarine has any
44

Receptors
significant effect on muscarinic receptors. By the same token, muscarine and atropine (compounds that activate and inhibit muscarinic receptors) are without effect on the processes regulated by nicotinic receptors.
The nicotinic receptors are ligand-gated ion channels and so are termed ionotropic receptors. They are members of the Cys-loop superfamily of channels (see below), which includes the 5-HT3 receptor (activated by 5-hydroxytryptamine, serotonin), the receptor for glycine and the receptors GABAA and GABAC (activated by -aminobutyric acid, GABA). Note however, that glycine and GABA receptors are anion-selective rather than cationselective channels, responding to ligands that cause electrical inhibition. For all of these, the receptor and ion channel functions are intrinsic properties of the same protein.
Muscarinic receptors
Whereas acetylcholine acts through the nicotinic receptor to stimulate contraction of skeletal muscle, it decreases the rate and force of contraction of heart muscle through muscarinic receptors. These belong to the seven transmembrane-spanning (7TM) superfamily of G protein-linked receptors and they exist as five subtypes, M1, M2, M3, m4, and m5. All five subtypes are present in the CNS. M1 receptors are expressed in ganglia and a number of secretory glands. M2 receptors are present in the myocardium and in smooth muscle and M3 receptors are also present in smooth muscle and in secretory glands (Table 3.2).
Muscarine and related compounds have many actions, dependent on the tissues to which they are applied and the receptor subtypes that are expressed. The structures and the mechanisms of action of the receptors activated by muscarine are closely related to those activated by the catecholamines. Like the adrenergic receptors, the diversity of their functions first became apparent with the development of a specific antagonist. This was pirenzepine, which blocks M1 receptors and prevents gastric acid secretion while being without effect on a number of other responses elicited by muscarinic receptors.
Although the muscarinic and nicotinic receptors share a common physiological stimulus, and although the muscarinic receptors can regulate ion fluxes indirectly (for example in the heart), they are not in themselves ion channels. The difference goes much further than this. Not only are these
receptors unrelated in terms of structure and evolution, but the conformation of the acetylcholine as it binds is likely to be different. While nicotine and muscarine are very different from one another (Figure 3.4) and contain ring structures that lend rigidity, the acetylcholine molecule is more flexible and is able to adopt different conformations that may be stabilized as it slots into its binding sites in the two receptors.
Tubocurarine is the main constituent of curare, a mixture of plant alkaloids obtained from Chondrodendron tomentosum or Strychnos toxifera, used as an arrow poison by some South American Indians. Claude Bernard (1856) showed that it causes paralysis by blocking neuromuscular transmission;8 Langley (1905) suggested that it acts by combining with the receptive substance at the motor endplate (see Chapter 1).9 Other related (synthetic) compounds such as gallamine differ mainly in their duration of action.
By convention, uppercase letters (M1, etc.) are used to assign receptors that have been pharmacologically defined, and lower-case letters (m4, etc.) are used
to assign those receptors that have been revealed only by molecular cloning.
45

Signal Transduction
Table 3.2 Muscarinic receptor subtypes
Subtype |
Antagonists |
Tissuea |
Transducer |
Effector |
M1 |
atropine, pirenzipine |
autonomic ganglia |
Gq |
phospholipase C |
|
|
|
|
(increases cytosol Ca2 ) |
M2 |
atropine, AF-DX 384b |
myocardium smooth |
Gi, Go |
activation of K |
|
|
muscle |
|
channels, inhibition of |
|
|
|
|
adenylyl cyclase |
|
|
|
|
|
M3 |
atropine |
smooth muscle |
Gq |
phospholipase C |
|
|
secretory glands |
|
(increases cytosol Ca2 ) |
m4 |
atropine, AF-DX 384b |
|
Gi, Go |
inhibition of adenylyl |
|
|
|
|
cyclase |
|
|
|
|
|
m5 |
atropine |
|
Gq |
phospholipase C |
|
|
|
|
(increases cytosol Ca2 ) |
aMultiple subtypes occur in many tissues. All are present in the CNS. This column indicates tissues with high levels of expression. bA benzodiazepine drug.
As with the adrenergic receptors, the response pathways operated by the muscarinic receptors for acetylcholine are all mediated through GTPbinding proteins. In the case of M1, M3, and probably the m5 subtype, the transduction is by G proteins of the Gq family, which causes activation of phospholipase C (PLC). This enzyme hydrolyses the phosphoinositide, phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2). One of the products is
inositol-1,4,5-trisphosphate (IP3) which releases Ca2 ions from intracellular Ca2 stores (for detailed discussion, see Chapter 7). The M2 and m4 receptors address G proteins of the Gi class with consequent inhibition of adenylyl cyclase and activation of K channels (see Chapter 5).
Nicotinic receptors are ion channels
Among the receptors and over the years the nicotinic cholinergic receptor has received the most detailed attention and in many respects is the best understood. There are particular reasons for this:
•
•
•
•
Specialized tissues exist in which nicotinic receptors are present in huge quantities.
There are toxins that bind specifically to the receptor with high affinity, enabling its isolation.
The channel properties of individual nicotinic receptor molecules are readily characterized by patch clamp electrophysiology. Three-dimensional structures of nicotinic receptors in situ have been obtained.
46
Receptors
Fig 3.4 Structures of cholinergic receptor agonists (red) and antagonists (black).
Electric organs provide a source
Tissues in which nicotinic receptors are abundant include the neuromuscular junction, where there may be as many as 4 107 copies on a single motor endplate of skeletal muscle (Figure 3.5). When acetylcholine is secreted from the presynaptic (prejunctional) membrane into the synaptic cleft, it binds
to receptors on the postsynaptic surface. This permits extracellular Na to flow through the nicotinic channels, down its concentration and voltage gradient into the muscle cell, causing a local depolarization that may lead ultimately to contraction. This abundance of receptors is vastly exceeded in the electric organs of the marine ray, Torpedo and the freshwater electric
47

Signal Transduction
Fig 3.5 -Bungarotoxin staining of nicotinic receptors at a neuromuscular junction: thin section electron micrographs of frog cutaneous pectoris muscle.
The nerve terminal, with mitochondria and numerous synaptic vesicles, is encased by a Schwann cell and abuts a muscle fibre. The preparation was treated with -bungarotoxin fused to horseradish peroxidase, followed by 3,3 -diaminobenzidine to reveal the acetylcholine receptors as an electrondense reaction product. The label is mainly confined to the extracellular
surface of the postjunctional membrane with some diffusion into the synaptic cleft. The scale bars are 0.5 m
From Burden et al.10
eel, Electrophorus. These electroplax organs are specialized developments of skeletal muscle and they express huge numbers of receptor molecules, about 2 1011 per endplate. As early as 1937, it was discovered that Torpedo electroplax could turn over more than its own weight of acetylcholine in an hour. The first demonstration of the biosynthesis of acetylcholine by a cellfree electroplax preparation was reported in 1944. Even today, the isolated postsynaptic membranes of the Torpedo electric organ provide an important source material for structural studies.
-Bungarotoxin, a toxic peptide obtained from the venom of the snake Bungarus multicinctus, binds at
the agonist binding site of neuromuscular-type acetylcholine receptors. There are similar snake venom -toxins that bind to the neuronal isoforms.
-Bungarotoxin blocks neuromuscular transmission
Because of their abundance in these specialized tissues it became possible to isolate, purify, and reconstitute nicotinic receptors, albeit in small quantities, well before the advent of cloning. The isolation was achieved by affinity chromatography using toxins that block neuromuscular transmission, in particular -bungarotoxin. This 8 kDa peptide binds specifically and with high affinity to nicotinic receptors (KD 10 12–10 9 mol l 1), with consequent inactivation. In the laboratory, the toxin can be immobilized by attachment to a solid phase (such as Sepharose). By applying to this affinity column
a solution of proteins released by detergent from muscle or electroplax membranes, it is possible to isolate receptor molecules.11–13
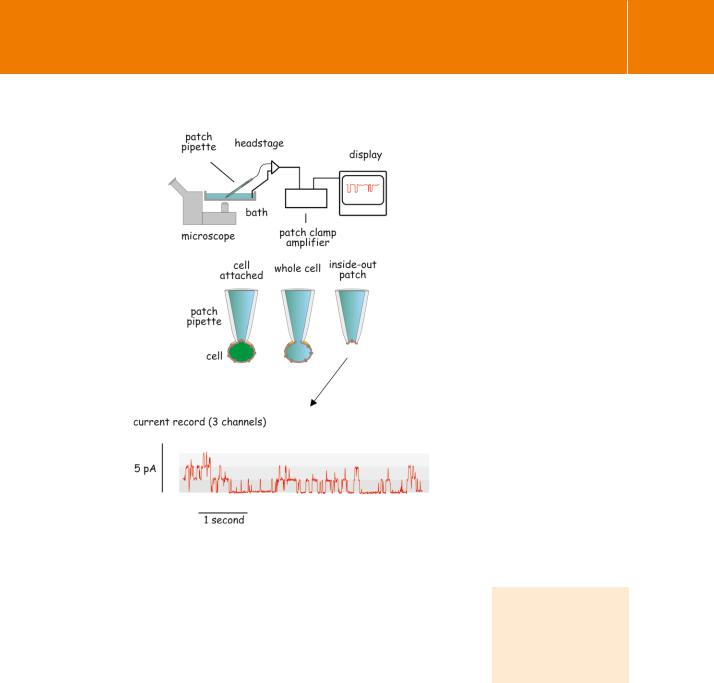
Patch clamp electrophysiology of single channels
With the introduction of the patch clamp technique by Neher and Sakmann,14 it became possible to study individual ion channels in situ in a membrane. It is hard to overestimate the contribution that this advance has made to our understanding of channel mechanisms. The idea itself is simple enough.
48
Receptors
A patch pipette, which is a glass microelectrode with a smooth (fire-polished) tip a few micrometres in diameter, is pressed gently against the outside of a cell. With gentle suction a very tight seal (typically 109 ) forms between the pipette tip and the cell membrane. A small area of membrane can then be removed from the cell without breaking the seal (Figure 3.6). The end result is either an outside-out or an inside-out patch of membrane. These contain only a very few ion channels, but depending on the orientation, the ligand binding sites are either exposed to the exterior or to the contents of the patch-pipette. The paucity of channels within the patch and the high resistance seal then enable the recording of membrane current at a controlled voltage. The recordings reveal individual channel openings and closures.
Nicotinic receptors were among the first channels to be characterized in this way. Like other ion channels they open and close abruptly in an all-or-none fashion and they do this spontaneously. The effect of acetylcholine is to increase the probability that a particular channel is in its open state.
Fig 3.6 Using membrane patches to record single channel opening and closure.
Typically, cells are allowed to adhere to the bottom of a shallow dish. Individual cells are then attached to a patch pipette. A typical current record obtained from a patch of membrane, detached from a cell, is shown.
Erwin Neher and Bert Sakmann shared the 1991 Nobel Prize for their discoveries concerning the function of single ion channels in cells.
49
