
- •Adrenaline (again)
- •Adrenergic receptor agonists and antagonists
- •Acetylcholine receptors
- •Acetylcholine
- •Cholinergic receptor subtypes
- •Nicotinic receptors
- •Muscarinic receptors
- •Nicotinic receptors are ion channels
- •Architecture of the nicotinic receptor
- •Other ligand-gated ion channels
- •The 7TM superfamily of G-protein-linked receptors
- •Categories of 7TM receptor
- •Receptor diversity: variation and specialization
- •Binding of low-molecular-mass ligands
- •Calcium sensors and metabotropic receptors
- •Proteinase-activated receptors (PARs)
- •The adhesion receptor subfamily
- •Frizzled
- •Receptor–ligand interaction and receptor activation
- •A two-state equilibrium description of receptor activation
- •Receptor dimerization
- •Transmitting signals into cells
- •The receptor and the effector: one and the same or separate entities?
- •Mixing and matching receptors and effectors
- •Intracellular 7TM receptor domains and signal transmission
- •Adrenaline (yet again)
- •References

Signal Transduction
Does it follow that dimerization provides an alternative signal for activation of the 2-adrenergic receptor? The picture becomes more complicated when receptors are visualized on the cell surface by labelling with membraneimpermeable dyes. The evidence points three ways. For a large number of 7TM receptors dimerization appears to be constitutive, unaffected by the presence of agonists. Examples of constitutively formed dimers include the2-adrenergic receptor, muscarinic receptors,83 the chemokine receptor CXCR284 (see page 494), and Frizzled 185 (see page 426). On the other hand, the extent of dimerization of the chemokine receptors CCR2, CXCR4, and CXCR586 increases in response to stimulation with agonists, and the dimer content of -opioid receptors actually diminishes. So while it is not possible to draw any general conclusions regarding a role of receptor homodimerization as a signal, there is little doubt that it is important in the processes of receptor maturation, folding, and trafficking to and from the cell surface, with dimer formation occurring early in the secretory pathway. For reviews of this field, see Hansen and Sheikh,87 Terrillon and Bouvier.88
Other receptors
For receptors of other types, dimerization is well established as the initiating step in the signalling processes that they control. These include receptors with intrinsic tyrosine kinase activity (e.g. growth factor receptors such as those for EGF, Chapter 12), with intrinsic serine/threonine kinase activity (Chapter 20), receptors which interact with cytosolic tyrosine kinases (Chapter 17), and receptors which are tightly linked to tyrosine kinases (cytokine receptors, Chapter 17).
Transmitting signals into cells
The receptor and the effector: one and the same or separate entities?
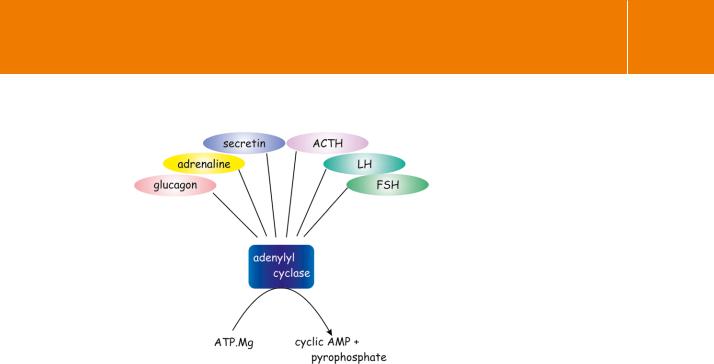
When it first became apparent that hormones induce enzymatic activity at the cell membrane, the receptors themselves were no more than concepts and ideas used to explain phenomena. It soon became evident that a receptor and its catalytic function might not be two facets of a single entity. For example, adipocytes have receptors for many different types of hormone. Adrenaline, ACTH, and glucagon all act to increase the concentration of intracellular cAMP. However, the maximal rate of cAMP formation due to a particular stimulus cannot be further enhanced by stimulation of a second receptor type.89,90 Other agents such as prostaglandins (PGE) and clonidine (an 2-adrenergic reagent), adenosine, and insulin, all acting through their own specific receptors, suppress the induction of cAMP synthesis. For these cells anyway, the inference is that multiple receptors can communicate with a common pool of adenylyl cyclase (Figure 3.19). From this it follows that the receptors are
70
Receptors
Fig 3.19 Multiple receptors coupled to a common pool of adenylyl cyclase.
A single pool of adenylyl cyclase can be accessed by many disparate hormone receptors. In general, when cyclase is maximally stimulated by one class of receptors further activation by ligands binding at other receptors is not possible. (There are however, some exceptions in which maximal activation of cyclase requires two types of receptor to act together in synergy: see Chapter 5.)
Adapted from Rodbell.91
likely to be discrete entities, each capable of communicating independently with the limited pool of enzyme.
To make things more complicated, there are situations in which two hormones are required together to cause stimulation. For example, in brain cortical slices the activation of adenylyl cyclase by noradrenaline
( -adrenergic stimulation which can be blocked by phentolamine, but not by propranolol) requires the simultaneous application of adenosine (blocked by theophylline).92 Here, with full activation requiring the involvement of two quite distinct receptors, one might want to infer that these are integral with the catalytic unit itself; (although there is a much better explanation for this, which is discussed in Chapter 5; see Figure 5.4, page 139).
Mixing and matching receptors and effectors
A direct demonstration of the independent existence of receptors and their downstream effector enzymes required a procedure by which they could be obtained separately and then reconstituted into a functioning unit. In precloning days, this presented a difficult problem since the receptors and the catalytic units which they control always share a common membrane location. Rather than attempt to separate them physically, the solution was to inhibit the cyclase selectively, while leaving the receptors intact, and then to use a second cellular source of cyclase, bringing the two together in order to achieve activity.
71
Signal Transduction
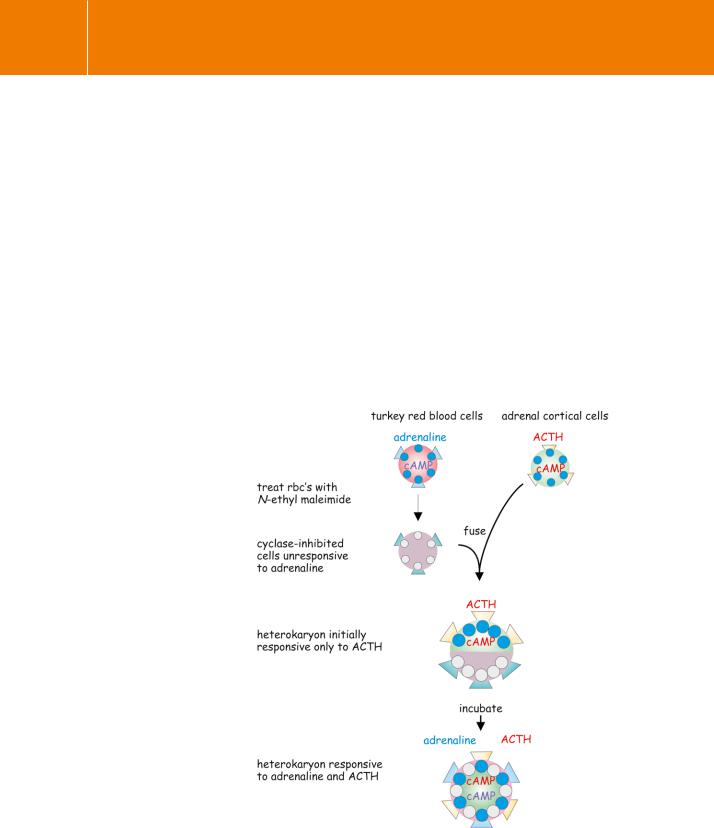
Fig 3.20 Evidence for independent lateral diffusion of receptors and adenylyl cyclase.
By this experiment, illustrated schematically, it was shown that hormone receptors and their effector enzymes (adenylyl cyclase) are separate molecular entities capable of independent lateral diffusion in the plane of a cell membrane. For details see text.
The alkylating agent N-ethylmaleimide was used to destroy the cyclase activity of turkey red blood cells while leaving their membrane receptors for adrenaline unimpaired. These cyclase-inactive cells were then fused to adrenal cortical cells as a source of the active enzyme (Figure 3.20). (Adrenal cortical cells lack catecholamine receptors but generate cAMP when treated
with the peptide hormone ACTH.) Immediately after the fusion cyclase could only be activated by ACTH, but over a period of a few hours the sensitivity to adrenaline recovered.93 This delay is commensurate with the rate at which the surface proteins of the two component cells diffuse laterally and merge with each other in the fused plasma membrane of the heterokaryon (the fused cell containing two nuclei). Since the cyclase to which the receptor for adrenaline is normally coupled had been irreversibly inactivated before the fusion, this could only mean that the -adrenergic receptors derived from the turkey red blood cells were now activating the adrenal cortical cell enzyme. It follows that the receptors and the catalytic units that they activate must be situated on different protein molecules. The experiment gave no indication that there
72
