
Photochemistry_of_Organic
.pdf
416 |
Chemistry of Excited Molecules |
Since oxygen is a highly symmetric molecule, the potential stereoselectivity of photooxygenation reaction should be largely controlled by the structure of the substrate, especially when a concerted or highly synchronous pathway is expected.1421 For example, formation of an endoperoxide stereoisomer 536 is selectively enhanced when a bulky silyl group prevents the attack of oxygen from the same face (Scheme 6.258).1446 Tetraphenylporphyrin (TPP) was utilized here to generate the singlet oxygen.
OBz |
|
|
OBz |
|
OBz |
|
OAc |
hν |
OAc |
|
OAc |
||
|
|
O |
+ |
O |
||
|
|
|
|
|||
OR |
TPP, O2 |
O |
|
O |
||
OR |
|
OR |
||||
0 oC, CCl |
|
|||||
|
|
|
|
|
||
|
|
4 |
536 |
|
|
|
|
|
|
|
|
|
|
R = -H: |
|
|
48 |
|
32% |
|
R = -Si(Me)2(t-Bu): |
78 |
|
2% |
|||
Scheme 6.258
Only activated aromatic compounds, such as benzenes with good electron-donating
substituents or polycyclic aromatic hydrocarbons, can undergo [4 þ 2] photooxygenation with singlet oxygen.1421,1425 For example,1O2 addition to 1,4-dimethylnaphthalene,
anthracene and pentacene proceeds with reaction rate constants on the order of 104, 105 and 109 mol 1 s 1, respectively, evidently increasing with increasing electron-donating abilities of the systems.1425 The mechanism is believed to be a concerted addition through a symmetrical transition state involving charge transfer from the aromatic compound to oxygen.1385 This addition is reversible; endoperoxides easily decompose either thermally to the starting aromatic compounds and singlet or ground state oxygen, or photochemically (Scheme 6.262).1387 Scheme 6.259 shows the photooxidation of heterocoerdianthrone (537); the resulting endoperoxide 538 can be deoxygenated by irradiation at 313 nm (F ¼ 0.27).218 This compound can be used as an actinometer.214
O |
|
O |
|
1O |
|
|
2 |
O |
|
hν, -1O2 |
O |
|
|
|
O |
|
O |
537 |
|
538 |
Scheme 6.259
Scheme 6.260 shows an example of the application of photooxygenation in the total synthesis of dysidiolide (539), a cdc25A protein phosphatase inhibitor, involving
regioselective oxidation of the furan moiety of 540 in nearly quantitative chemical yield in the last step of the synthetic procedure.1447
Molecular Oxygen |
419 |
6.7.3Singlet Oxygen: ene Reaction
3O2, sens
Recommended review articles.135,136,1358,1359,1376,1377,1422,1423,1450
Selected theoretical and computational photochemistry references.1428,1429,1451–1453
The ene or Schenck1454 reaction is another type of photooxygenation reaction that
involves singlet oxygen (Section 6.7.1) and an alkene containing at least one allylic hydrogen.1376,1377,1422,1423,1450 The reaction intermediates proposed for the [2 þ 2]
and [4 þ 2] photooxygenation (Section 6.7.2; Scheme 6.250) can also be considered for the ene reactions (Scheme 6.264); nevertheless, highly negative activation entropies may indicate a concerted mechanism1455 or an exciplex 550 involvement.1456 There
is also strong evidence that a perepoxide intermediate 551 is formed in certain
cases.1450,1456–1459
|
|
|
|
1O |
HOO |
|
|
|
|
+ |
|
||
|
|
|
|
|
2 |
|
|
|
|
CH3 |
|
concerted |
CH2 |
|
|
|
|
|
stepwise |
|
|
|
δ |
|
|
O |
O |
δ |
1O2 or |
O O |
or |
O or |
O |
|
|
CH3 |
H3C |
|
|
|
|
|
550 |
551 |
|
|
|
|
|
|
|
|
|
||
Scheme 6.264
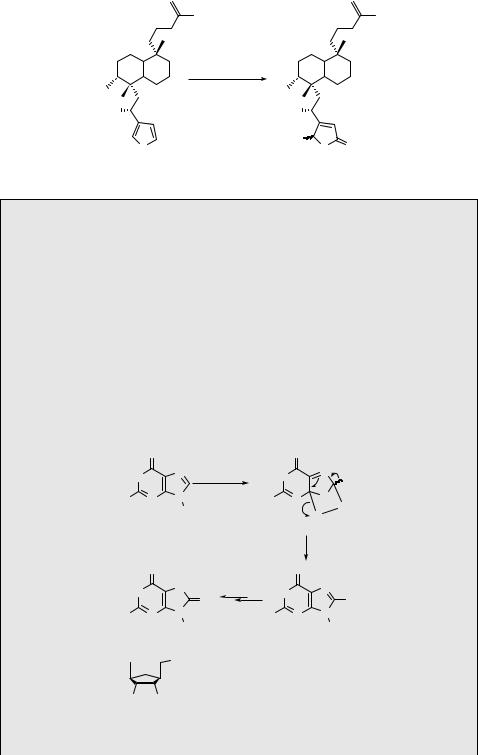
Competitive [4 þ 2] and ene reactions of 2,4-dimethylpenta-1,3-diene (552) were suggested to occur by either a concerted or a perepoxide intermediate pathway (Scheme 6.265).1460 The major [4 þ 2] product 553 is formed in nonpolar solvents nearly exclusively, whereas formation of the ene product (554) via a polar perepoxide intermediate 555 is enhanced in polar solvents, such as methanol. The less stable s-cis-conformation of 552 is required for the concerted process. Another competing process, physical quenching of singlet oxygen, was found to be at least as efficient as the chemical processes.
A concerted pathway for ene reaction was ruled out by a stereochemical investigation of photooxygenation of an optically pure, deuterated 3,4-dimethyl-1-phenylpent-2-ene (556) (Scheme 6.266).1461 The reaction was suggested to proceed suprafacially and
420 |
Chemistry of Excited Molecules |
|
||
|
1O |
O |
|
|
|
2 |
|
|
|
|
|
O |
|
|
|
|
553 |
O |
|
|
|
|
|
|
|
|
|
O |
HOO |
|
|
1O |
2 |
|
|
s-cis-552 |
|
|
|
|
|
s-trans-552 |
555 |
554 |
Scheme 6.265
|
O |
D |
|
|
|
|
O |
Ph |
|
HO |
Ph |
|
|
|
|||
|
|
|
|
|
|
|
|
H |
[H] |
|
H |
1O2 |
|
|
|
||
557 |
|
|
559 |
||
Ph |
|
|
|
|
|
H D |
|
D Ph |
|
|
Ph |
556 |
|
|
|
HO |
|
O |
H |
|
|
||
|
[H] |
H |
|||
|
|
O |
|
||
|
|
|
|
|
|
|
558 |
|
|
560 |
|
Scheme 6.266
stereospecifically via perepoxide intermediates 557 and 558 (or exciplex species), in which the bulky phenyl group is kept away from the reaction centre and either a hydrogen or deuterium atom is abstracted. Because no stereochemical (the product concentration
ratio [559]:[560] was equal to 1) and no isotopic (kH/kD ¼ 1) discrimination was observed, the rate-determining step must be the attack of singlet oxygen on the double bond.1377 A
reduction step ([H]) is necessary to give the corresponding alcohols instead of primary hydroperoxides.
Both steric and electronic effects can influence the regioselectivity of ene reactions. For example, a strong preference for hydrogen abstraction from the more substituted side of
the double bond of the perepoxide intermediates, generated from trisubstituted alkenes, is observed.1376,1377 This effect has been called the cis effect and explained on the basis of
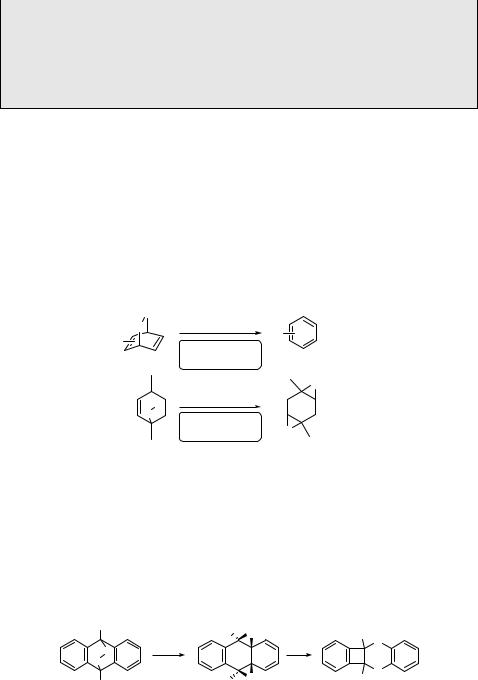
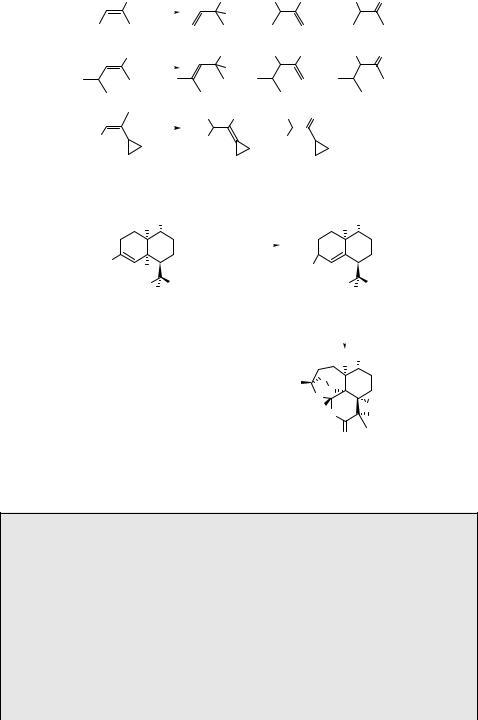
orbital interactions1423 and activation entropy differences.1457 Scheme 6.267 shows the product distribution after photooxygenation of three alkenes (561–563).1461 There is an apparent steric effect of the methyl versus 2-propyl substituents in the first two cases. The cyclopropyl moiety in the last example remains unreacted, which rules out the formation of a biradical intermediate (see also Special Topic 6.10).
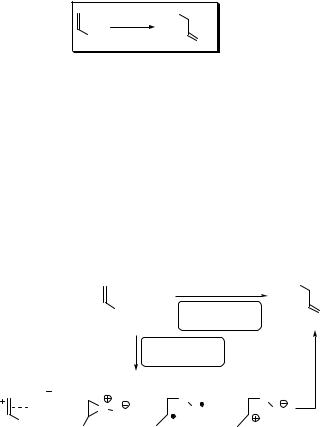
Scheme 6.268 shows the synthesis of the antimalarial drug artemisinin (564) from dihydroartemisinic acid (565): photooxygenation of 565 in the presence of Methylene Blue gives 566, which is oxidized by ground-state oxygen in 30% chemical yield.1462
|
|
|
Molecular Oxygen |
|
|
421 |
|||||||
CD3 |
1O |
|
OOH |
HOO |
|
CD3 |
HOO |
CD2 |
|||||
|
2 |
|
|
CD3 |
+ |
|
|
|
|
|
+ |
|
|
561 |
|
|
|
|
|
|
|
|
|
|
|||
|
|
53% |
|
|
40% |
|
|
|
|
7% |
|||
CD3 |
1O |
|
OOH |
HOO |
|
CD3 |
HOO |
CD2 |
|||||
|
2 |
|
|
CD3 |
+ |
|
|
|
|
|
+ |
|
|
562 |
|
|
|
|
|
|
|
|
|
|
|||
|
|
50% |
|
|
43% |
|
|
|
|
7% |
|||
|
1O |
HOO CD3 |
HOO CD2 |
|
|||||||||
|
2 |
|
|
|
+ |
|
|
|
|
|
|
|
|
MeO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MeO |
MeO |
|
|
|
||||||
563 |
|
|
72% |
|
|
28% |
|
|
|
|
|
||
|
|
|
|
Scheme 6.267 |
|
|
|
|
|
|
|
||
H |
CH3 |
|
|
|
|
|
|
|
|
|
CH3 |
||
|
|
|
|
|
|
|
|
|
|
H |
|
||
|
|
|
|
hν |
|
|
|
|
|
|
|
|
|
|
|
|
Methylene Blue, O |
H3C |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|||||||
H3C |
|
|
|
|
2 |
HOO |
|
|
|
||||
H |
|
|
|
|
|
|
|
|
|
||||
HOOC |
CH3 |
|
|
|
|
HOOC |
CH3 |
||||||
|
H |
|
|
|
|
|
|
|
|
|
H |
|
|
565 |
|
|
|
|
|
|
|
|
|
566 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3O |
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3 |
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
H3C |
O |
|
||||
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
O O |
H |
|||
|
|
|
|
|
|
|
|
|
H O |
||||
|
|
|
|
|
|
|
|
|
H |
||||
O
564
Scheme 6.268
Case Study 6.37: Solid support synthesis – ene reaction in zeolites
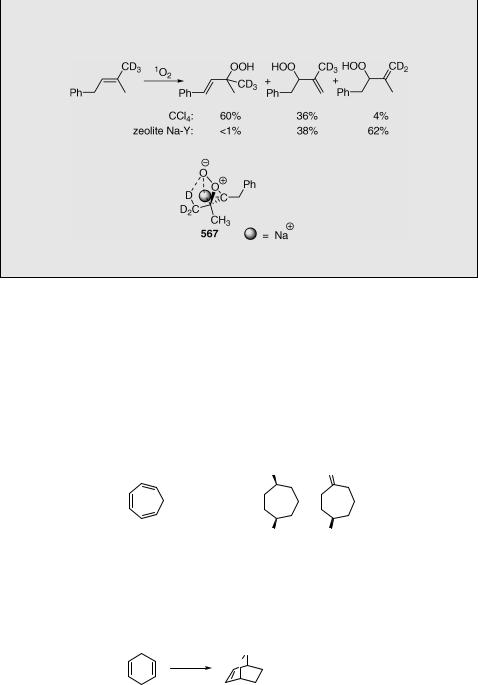
Contrary to reactions in solution (see the preceding text), the less substituted side of some trisubstituted alkenes becomes more vulnerable to ene photooxygenation if it takes place in thionin-supported zeolite Na-Y (Scheme 6.269).1463 Synchronous stabilization (less hindered) interaction of the alkali metal cation (Naþ ; in zeolite) with the former C¼C bond of the alkene and oxygen during perepoxide formation (567) is apparently responsible for this anomalous behaviour. Thionin sensitizer used to generate singlet oxygen in the solid support was placed within the zeolite via an aqueous-based cation-exchange process.1464
Experimental details.1463 Thionin-exchanged zeolite (1 g; prepared according to the literature;1464 the loading level was lower than one dye molecule per 100 supercages)
Photosensitizers, Photoinitiators and Photocatalysts |
423 |
3.Predict the major photoproduct(s):
(a)
hν
Rose Bengal, O2
CH2Cl2, MeOH
[ref. 1467]
(b)
O |
hν |
(light emission |
|
||
|
Rose Bengal, O2 |
in the final step) |
|
|
[ref. 1468]
6.8 Photosensitizers, Photoinitiators and Photocatalysts
We have encountered examples of photochemically activated, indirect initiation of chemical reactions via energy or electron transfer many times throughout the text. It has been shown that they require the utilization of specific reaction conditions and experimental arrangements. They are extremely useful in many laboratory experiments and also for practical photochemical applications, especially when direct excitation by light is difficult to accomplish or unwanted side-reactions take place. Also, such reactions can be very effective, so they can be successfully implemented when a high degree of reactant degradation is needed. In Section 6.8.1, various applications of sensitization (Table 6.21, entry 1) and also photoinduced electron transfer techniques (entry 2) are presented, involving organic photosensitizers, photocatalysts and photoinitiators for organic synthesis, biology or material/surface sciences. Both excited photosensitizer and photocatalyst are typically regenerated in processes such as deactivation or electron transfer, whereas photoinitiators are generally consumed in the reaction.
Electron transfer processes involving excited transition metal photocatalysts are examined in Section 6.8.2. Reactive radical ions are usually generated from neutral
Table 6.21 Photoprocesses involving photosensitizers, photocatalysts and photoinitiators
Entry |
Starting materiala |
Product(s)a |
|
Process |
Section |
||||||
1 |
R |
þ |
sensitizer |
. |
þ . |
|
|
Sensitization |
6.8.1 |
||
|
R |
|
|
sensitizer |
|||||||
2 |
D |
|
n |
D |
þ |
þ A |
|
|
Photoinduced electron transfer |
6.8.1 |
|
3þ A þ h |
2 |
|
. |
þ H þ |
|||||||
3 |
Fe þ þ H2O þ hn |
Fe. |
|
þ þ HO. |
Homogeneous photocatalysis |
6.8.2 |
|||||
4 |
D þ A þ TiO2 þ hn |
D |
þ þ A þ TiO2 |
Heterogeneous photocatalysis |
6.8.2 |
||||||
aD ¼ electron donor; A ¼ electron acceptor.
424 |
Chemistry of Excited Molecules |
organic molecules, which then undergo subsequent secondary transformations. Photoredox photo-Fenton s process (entry 3) is an example of the catalysis in a homogeneous solution. A photocatalytic process, utilizing insoluble excited semiconductor such as titanium dioxide which promote interfacial electron transfer between the excited semiconductor surface and adsorbed molecules, is portrayed in entry 4.
6.8.1Organic Photosensitizers, Photocatalysts and Photoinitiators
sens |
hν |
sens * |
sens |
sens |
hν |
sens |
|
sens * |
|||||
|
|
R |
R * |
|
R |
R |
sens hν sens* sens-H R-H R
sens-H R-H R
Recommended review articles.602,1134,1469–1471
Organic Photosensitizers: Energy Transfer
Most of the chemical processes described in the previous sections occur directly from an excited state precursor (R), which can be obtained either directly by absorption of light (Scheme 6.270a) or indirectly by photosensitization (via energy transfer; Scheme 6.270b). In the latter case, the sensitizer (sens) is regenerated as a ground-state molecule and therefore it must be electronically excited again in order to be involved in a new sensitization cycle.
(a)R  R*
R*  product(s)
product(s)
|
|
direct |
chemical |
|
irradiation |
reaction |
|
(b) |
sens * |
sens |
|
R |
|
R * |
product(s) |
|
sensitization |
chemical |
|
|
reaction |
||
|
|
|
|
Scheme 6.270
Photosensitization is one of the most common and practical ways to generate excited states, particularly triplet states, especially when excitation at a desired wavelength cannot be achieved or when it does not lead to the desired excited state (Section 2.2). Good photosensitizers should absorb strongly in the region of interest and have sufficient excited-state lifetimes. For efficient energy transfer, the process should be exergonic. Table 6.22 lists some of the common photosensitizers and their typical applications.

 O
O  +
+ OOH
OOH
