
- •CONTENTS
- •Preface
- •Contributors
- •1 Introduction to Toxicology
- •1.1 Definition and Scope, Relationship to Other Sciences, and History
- •1.1.2 Relationship to Other Sciences
- •1.1.3 A Brief History of Toxicology
- •1.3 Sources of Toxic Compounds
- •1.3.1 Exposure Classes
- •1.3.2 Use Classes
- •1.4 Movement of Toxicants in the Environment
- •Suggested Reading
- •2.1 Introduction
- •2.2 Cell Culture Techniques
- •2.2.1 Suspension Cell Culture
- •2.2.2 Monolayer Cell Culture
- •2.2.3 Indicators of Toxicity in Cultured Cells
- •2.3 Molecular Techniques
- •2.3.1 Molecular Cloning
- •2.3.2 cDNA and Genomic Libraries
- •2.3.3 Northern and Southern Blot Analyses
- •2.3.4 Polymerase Chain Reaction (PCR)
- •2.3.5 Evaluation of Gene Expression, Regulation, and Function
- •2.4 Immunochemical Techniques
- •Suggested Reading
- •3.1 Introduction
- •3.2 General Policies Related to Analytical Laboratories
- •3.2.1 Standard Operating Procedures (SOPs)
- •3.2.2 QA/QC Manuals
- •3.2.3 Procedural Manuals
- •3.2.4 Analytical Methods Files
- •3.2.5 Laboratory Information Management System (LIMS)
- •3.3 Analytical Measurement System
- •3.3.1 Analytical Instrument Calibration
- •3.3.2 Quantitation Approaches and Techniques
- •3.4 Quality Assurance (QA) Procedures
- •3.5 Quality Control (QC) Procedures
- •3.6 Summary
- •Suggested Reading
- •4 Exposure Classes, Toxicants in Air, Water, Soil, Domestic and Occupational Settings
- •4.1 Air Pollutants
- •4.1.1 History
- •4.1.2 Types of Air Pollutants
- •4.1.3 Sources of Air Pollutants
- •4.1.4 Examples of Air Pollutants
- •4.1.5 Environmental Effects
- •4.2 Water and Soil Pollutants
- •4.2.1 Sources of Water and Soil Pollutants
- •4.2.2 Examples of Pollutants
- •4.3 Occupational Toxicants
- •4.3.1 Regulation of Exposure Levels
- •4.3.2 Routes of Exposure
- •4.3.3 Examples of Industrial Toxicants
- •Suggested Reading
- •5 Classes of Toxicants: Use Classes
- •5.1 Introduction
- •5.2 Metals
- •5.2.1 History
- •5.2.2 Common Toxic Mechanisms and Sites of Action
- •5.2.3 Lead
- •5.2.4 Mercury
- •5.2.5 Cadmium
- •5.2.6 Chromium
- •5.2.7 Arsenic
- •5.2.8 Treatment of Metal Poisoning
- •5.3 Agricultural Chemicals (Pesticides)
- •5.3.1 Introduction
- •5.3.3 Organochlorine Insecticides
- •5.3.4 Organophosphorus Insecticides
- •5.3.5 Carbamate Insecticides
- •5.3.6 Botanical Insecticides
- •5.3.7 Pyrethroid Insecticides
- •5.3.8 New Insecticide Classes
- •5.3.9 Herbicides
- •5.3.10 Fungicides
- •5.3.11 Rodenticides
- •5.3.12 Fumigants
- •5.3.13 Conclusions
- •5.4 Food Additives and Contaminants
- •5.5 Toxins
- •5.5.1 History
- •5.5.2 Microbial Toxins
- •5.5.3 Mycotoxins
- •5.5.4 Algal Toxins
- •5.5.5 Plant Toxins
- •5.5.6 Animal Toxins
- •5.6 Solvents
- •5.7 Therapeutic Drugs
- •5.8 Drugs of Abuse
- •5.9 Combustion Products
- •5.10 Cosmetics
- •Suggested Reading
- •6 Absorption and Distribution of Toxicants
- •6.1 Introduction
- •6.2 Cell Membranes
- •6.3 Mechanisms of Transport
- •6.3.1 Passive Diffusion
- •6.4 Physicochemical Properties Relevant to Diffusion
- •6.4.1 Ionization
- •6.5 Routes of Absorption
- •6.5.1 Extent of Absorption
- •6.5.2 Gastrointestinal Absorption
- •6.5.3 Dermal Absorption
- •6.5.4 Respiratory Penetration
- •6.6 Toxicant Distribution
- •6.6.1 Physicochemical Properties and Protein Binding
- •6.7 Toxicokinetics
- •Suggested Reading
- •7 Metabolism of Toxicants
- •7.1 Introduction
- •7.2 Phase I Reactions
- •7.2.4 Nonmicrosomal Oxidations
- •7.2.5 Cooxidation by Cyclooxygenases
- •7.2.6 Reduction Reactions
- •7.2.7 Hydrolysis
- •7.2.8 Epoxide Hydration
- •7.2.9 DDT Dehydrochlorinase
- •7.3 Phase II Reactions
- •7.3.1 Glucuronide Conjugation
- •7.3.2 Glucoside Conjugation
- •7.3.3 Sulfate Conjugation
- •7.3.4 Methyltransferases
- •7.3.7 Acylation
- •7.3.8 Phosphate Conjugation
- •Suggested Reading
- •8 Reactive Metabolites
- •8.1 Introduction
- •8.2 Activation Enzymes
- •8.3 Nature and Stability of Reactive Metabolites
- •8.4 Fate of Reactive Metabolites
- •8.4.1 Binding to Cellular Macromolecules
- •8.4.2 Lipid Peroxidation
- •8.4.3 Trapping and Removal: Role of Glutathione
- •8.5 Factors Affecting Toxicity of Reactive Metabolites
- •8.5.1 Levels of Activating Enzymes
- •8.5.2 Levels of Conjugating Enzymes
- •8.5.3 Levels of Cofactors or Conjugating Chemicals
- •8.6 Examples of Activating Reactions
- •8.6.1 Parathion
- •8.6.2 Vinyl Chloride
- •8.6.3 Methanol
- •8.6.5 Carbon Tetrachloride
- •8.6.8 Acetaminophen
- •8.6.9 Cycasin
- •8.7 Future Developments
- •Suggested Reading
- •9.1 Introduction
- •9.2 Nutritional Effects
- •9.2.1 Protein
- •9.2.2 Carbohydrates
- •9.2.3 Lipids
- •9.2.4 Micronutrients
- •9.2.5 Starvation and Dehydration
- •9.2.6 Nutritional Requirements in Xenobiotic Metabolism
- •9.3 Physiological Effects
- •9.3.1 Development
- •9.3.2 Gender Differences
- •9.3.3 Hormones
- •9.3.4 Pregnancy
- •9.3.5 Disease
- •9.3.6 Diurnal Rhythms
- •9.4 Comparative and Genetic Effects
- •9.4.1 Variations Among Taxonomic Groups
- •9.4.2 Selectivity
- •9.4.3 Genetic Differences
- •9.5 Chemical Effects
- •9.5.1 Inhibition
- •9.5.2 Induction
- •9.5.3 Biphasic Effects: Inhibition and Induction
- •9.6 Environmental Effects
- •9.7 General Summary and Conclusions
- •Suggested Reading
- •10 Elimination of Toxicants
- •10.1 Introduction
- •10.2 Transport
- •10.3 Renal Elimination
- •10.4 Hepatic Elimination
- •10.4.2 Active Transporters of the Bile Canaliculus
- •10.5 Respiratory Elimination
- •10.6 Conclusion
- •Suggested Reading
- •11 Acute Toxicity
- •11.1 Introduction
- •11.2 Acute Exposure and Effect
- •11.3 Dose-response Relationships
- •11.4 Nonconventional Dose-response Relationships
- •11.5 Mechanisms of Acute Toxicity
- •11.5.1 Narcosis
- •11.5.2 Acetylcholinesterase Inhibition
- •11.5.3 Ion Channel Modulators
- •11.5.4 Inhibitors of Cellular Respiration
- •Suggested Reading
- •12 Chemical Carcinogenesis
- •12.1 General Aspects of Cancer
- •12.2 Human Cancer
- •12.2.1 Causes, Incidence, and Mortality Rates of Human Cancer
- •12.2.2 Known Human Carcinogens
- •12.3 Classes of Agents Associated with Carcinogenesis
- •12.3.2 Epigenetic Agents
- •12.4 General Aspects of Chemical Carcinogenesis
- •12.5 Initiation-Promotion Model for Chemical Carcinogenesis
- •12.6 Metabolic Activation of Chemical Carcinogens and DNA Adduct Formation
- •12.7 Oncogenes
- •12.8 Tumor Suppressor Genes
- •12.8.1 Inactivation of Tumor Suppressor Genes
- •12.8.2 p53 Tumor Suppressor Gene
- •12.9 General Aspects of Mutagenicity
- •12.10 Usefulness and Limitations of Mutagenicity Assays for the Identification of Carcinogens
- •Suggested Reading
- •13 Teratogenesis
- •13.1 Introduction
- •13.2 Principles of Teratology
- •13.3 Mammalian Embryology Overview
- •13.4 Critical Periods
- •13.5 Historical Teratogens
- •13.5.1 Thalidomide
- •13.5.2 Accutane (Isotetrinoin)
- •13.5.3 Diethylstilbestrol (DES)
- •13.5.4 Alcohol
- •13.6 Testing Protocols
- •13.6.1 FDA Guidelines for Reproduction Studies for Safety Evaluation of Drugs for Human Use
- •13.6.3 Alternative Test Methods
- •13.7 Conclusions
- •Suggested Reading
- •14 Hepatotoxicity
- •14.1 Introduction
- •14.1.1 Liver Structure
- •14.1.2 Liver Function
- •14.2 Susceptibility of the Liver
- •14.3 Types of Liver Injury
- •14.3.1 Fatty Liver
- •14.3.2 Necrosis
- •14.3.3 Apoptosis
- •14.3.4 Cholestasis
- •14.3.5 Cirrhosis
- •14.3.6 Hepatitis
- •14.3.7 Oxidative Stress
- •14.3.8 Carcinogenesis
- •14.4 Mechanisms of Hepatotoxicity
- •14.5 Examples of Hepatotoxicants
- •14.5.1 Carbon Tetrachloride
- •14.5.2 Ethanol
- •14.5.3 Bromobenzene
- •14.5.4 Acetaminophen
- •14.6 Metabolic Activation of Hepatotoxicants
- •Suggested Reading
- •15 Nephrotoxicity
- •15.1 Introduction
- •15.1.1 Structure of the Renal System
- •15.1.2 Function of the Renal System
- •15.2 Susceptibility of the Renal System
- •15.3 Examples of Nephrotoxicants
- •15.3.1 Metals
- •15.3.2 Aminoglycosides
- •15.3.3 Amphotericin B
- •15.3.4 Chloroform
- •15.3.5 Hexachlorobutadiene
- •Suggested Reading
- •16 Toxicology of the Nervous System
- •16.1 Introduction
- •16.2 The Nervous system
- •16.2.1 The Neuron
- •16.2.2 Neurotransmitters and their Receptors
- •16.2.3 Glial Cells
- •16.3 Toxicant Effects on the Nervous System
- •16.3.1 Structural Effects of Toxicants on Neurons
- •16.3.2 Effects of Toxicants on Other Cells
- •16.4 Neurotoxicity Testing
- •16.4.1 In vivo Tests of Human Exposure
- •16.4.2 In vivo Tests of Animal Exposure
- •16.4.3 In vitro Neurochemical and Histopathological End Points
- •16.5 Summary
- •Suggested Reading
- •17 Endocrine System
- •17.1 Introduction
- •17.2 Endocrine System
- •17.2.1 Nuclear Receptors
- •17.3 Endocrine Disruption
- •17.3.1 Hormone Receptor Agonists
- •17.3.2 Hormone Receptor Antagonists
- •17.3.3 Organizational versus Activational Effects of Endocrine Toxicants
- •17.3.4 Inhibitors of Hormone Synthesis
- •17.3.5 Inducers of Hormone Clearance
- •17.3.6 Hormone Displacement from Binding Proteins
- •17.4 Incidents of Endocrine Toxicity
- •17.4.1 Organizational Toxicity
- •17.4.2 Activational Toxicity
- •17.4.3 Hypothyroidism
- •17.5 Conclusion
- •Suggested Reading
- •18 Respiratory Toxicity
- •18.1 Introduction
- •18.1.1 Anatomy
- •18.1.2 Cell Types
- •18.1.3 Function
- •18.2 Susceptibility of the Respiratory System
- •18.2.1 Nasal
- •18.2.2 Lung
- •18.3 Types of Toxic Response
- •18.3.1 Irritation
- •18.3.2 Cell Necrosis
- •18.3.3 Fibrosis
- •18.3.4 Emphysema
- •18.3.5 Allergic Responses
- •18.3.6 Cancer
- •18.3.7 Mediators of Toxic Responses
- •18.4 Examples of Lung Toxicants Requiring Activation
- •18.4.1 Introduction
- •18.4.2 Monocrotaline
- •18.4.3 Ipomeanol
- •18.4.4 Paraquat
- •18.5 Defense Mechanisms
- •Suggested Reading
- •19 Immunotoxicity
- •19.1 Introduction
- •19.2 The Immune System
- •19.3 Immune Suppression
- •19.4 Classification of Immune-Mediated Injury (Hypersensitivity)
- •19.5 Effects of Chemicals on Allergic Disease
- •19.5.1 Allergic Contact Dermatitis
- •19.5.2 Respiratory Allergens
- •19.5.3 Adjuvants
- •19.6 Emerging Issues: Food Allergies, Autoimmunity, and the Developing Immune System
- •Suggested Reading
- •20 Reproductive System
- •20.1 Introduction
- •20.2 Male Reproductive Physiology
- •20.3 Mechanisms and Targets of Male Reproductive Toxicants
- •20.3.1 General Mechanisms
- •20.3.2 Effects on Germ Cells
- •20.3.3 Effects on Spermatogenesis and Sperm Quality
- •20.3.4 Effects on Sexual Behavior
- •20.3.5 Effects on Endocrine Function
- •20.4 Female Reproductive Physiology
- •20.5 Mechanisms and Targets of Female Reproductive Toxicants
- •20.5.1 Tranquilizers, Narcotics, and Social Drugs
- •20.5.2 Endocrine Disruptors (EDs)
- •20.5.3 Effects on Germ Cells
- •20.5.4 Effects on the Ovaries and Uterus
- •20.5.5 Effects on Sexual Behavior
- •Suggested Reading
- •21 Toxicity Testing
- •21.1 Introduction
- •21.2 Experimental Administration of Toxicants
- •21.2.1 Introduction
- •21.2.2 Routes of Administration
- •21.3 Chemical and Physical Properties
- •21.4 Exposure and Environmental Fate
- •21.5 In vivo Tests
- •21.5.1 Acute and Subchronic Toxicity Tests
- •21.5.2 Chronic Tests
- •21.5.3 Reproductive Toxicity and Teratogenicity
- •21.5.4 Special Tests
- •21.6 In vitro and Other Short-Term Tests
- •21.6.1 Introduction
- •21.6.2 Prokaryote Mutagenicity
- •21.6.3 Eukaryote Mutagenicity
- •21.6.4 DNA Damage and Repair
- •21.6.5 Chromosome Aberrations
- •21.6.6 Mammalian Cell Transformation
- •21.6.7 General Considerations and Testing Sequences
- •21.7 Ecological Effects
- •21.7.1 Laboratory Tests
- •21.7.2 Simulated Field Tests
- •21.7.3 Field Tests
- •21.8 Risk Analysis
- •21.9 The Future of Toxicity Testing
- •Suggested Reading
- •22 Forensic and Clinical Toxicology
- •22.1 Introduction
- •22.2 Foundations of Forensic Toxicology
- •22.3 Courtroom Testimony
- •22.4.1 Documentation Practices
- •22.4.2 Considerations for Forensic Toxicological Analysis
- •22.4.3 Drug Concentrations and Distribution
- •22.5 Laboratory Analyses
- •22.5.1 Colorimetric Screening Tests
- •22.5.2 Thermal Desorption
- •22.5.6 Enzymatic Immunoassay
- •22.6 Analytical Schemes for Toxicant Detection
- •22.7 Clinical Toxicology
- •22.7.1 History Taking
- •22.7.2 Basic Operating Rules in the Treatment of Toxicosis
- •22.7.3 Approaches to Selected Toxicoses
- •Suggested Reading
- •23 Prevention of Toxicity
- •23.1 Introduction
- •23.2 Legislation and Regulation
- •23.2.1 Federal Government
- •23.2.2 State Governments
- •23.2.3 Legislation and Regulation in Other Countries
- •23.3 Prevention in Different Environments
- •23.3.1 Home
- •23.3.2 Workplace
- •23.3.3 Pollution of Air, Water, and Land
- •23.4 Education
- •Suggested Reading
- •24 Human Health Risk Assessment
- •24.1 Introduction
- •24.2 Risk Assessment Methods
- •24.2.2 Exposure Assessment
- •24.2.3 Dose Response and Risk Characterization
- •24.3 Noncancer Risk Assessment
- •24.3.1 Default Uncertainty and Modifying Factors
- •24.3.2 Derivation of Developmental Toxicant RfD
- •24.3.3 Determination of RfD and RfC of Naphthalene with the NOAEL Approach
- •24.3.4 Benchmark Dose Approach
- •24.3.5 Determination of BMD and BMDL for ETU
- •24.3.6 Quantifying Risk for Noncarcinogenic Effects: Hazard Quotient
- •24.3.7 Chemical Mixtures
- •24.4 Cancer Risk Assessment
- •24.5 PBPK Modeling
- •Suggested Reading
- •25 Analytical Methods in Toxicology
- •25.1 Introduction
- •25.2 Chemical and Physical Methods
- •25.2.1 Sampling
- •25.2.2 Experimental Studies
- •25.2.3 Forensic Studies
- •25.2.4 Sample Preparation
- •25.2.6 Spectroscopy
- •25.2.7 Other Analytical Methods
- •Suggested Reading
- •26 Basics of Environmental Toxicology
- •26.1 Introduction
- •26.2 Environmental Persistence
- •26.2.1 Abiotic Degradation
- •26.2.2 Biotic Degradation
- •26.2.3 Nondegradative Elimination Processes
- •26.3 Bioaccumulation
- •26.4 Toxicity
- •26.4.1 Acute Toxicity
- •26.4.2 Mechanisms of Acute Toxicity
- •26.4.3 Chronic Toxicity
- •26.4.5 Abiotic and Biotic Interactions
- •26.5 Conclusion
- •Suggested Reading
- •27.1 Introduction
- •27.2 Sources of Toxicants to the Environment
- •27.3 Transport Processes
- •27.3.1 Advection
- •27.3.2 Diffusion
- •27.4 Equilibrium Partitioning
- •27.5 Transformation Processes
- •27.5.1 Reversible Reactions
- •27.5.2 Irreversible Reactions
- •27.6 Environmental Fate Models
- •Suggested Reading
- •28 Environmental Risk Assessment
- •28.1 Introduction
- •28.2 Formulating the Problem
- •28.2.1 Selecting Assessment End Points
- •28.2.2 Developing Conceptual Models
- •28.2.3 Selecting Measures
- •28.3 Analyzing Exposure and Effects Information
- •28.3.1 Characterizing Exposure
- •28.3.2 Characterizing Ecological Effects
- •28.4 Characterizing Risk
- •28.4.1 Estimating Risk
- •28.4.2 Describing Risk
- •28.5 Managing Risk
- •Suggested Reading
- •29 Future Considerations for Environmental and Human Health
- •29.1 Introduction
- •29.2 Risk Management
- •29.3 Risk Assessment
- •29.4 Hazard and Exposure Assessment
- •29.5 In vivo Toxicity
- •29.6 In vitro Toxicity
- •29.7 Biochemical and Molecular Toxicology
- •29.8 Development of Selective Toxicants
- •Glossary
- •Index

306 ENDOCRINE SYSTEM
suggestions that the SHBG-receptor pathway is involved in this disease condition. The susceptibility of these membrane-bound receptor pathways in endocrine toxicity has received little attention, though conceivably toxicants could perturb these pathways by competing with endogenous hormone for binding to SHBG, resulting in the loss of stimulatory activity (antagonists) or inappropriate stimulation of activity (agonists).
17.3ENDOCRINE DISRUPTION
Xenobiotics have the ability to disrupt hormone activity through a variety of mechanisms, though the predominant mechanisms appear to involve binding to the hormone receptor, either as an agonist or antagonist, or by modulating endogenous steroid hormone levels.
17.3.1Hormone Receptor Agonists
A hormone receptor agonist is defined as a compound that binds to and activates a hormone receptor. Endogenous hormones function as agonists to their respective receptors. Xenobiotics can act as receptor agonists and stimulate receptor-dependent physiological processes in the absence of the endogenous receptor ligand (hormone). Such inappropriate stimulation can result in the errant expression of hormone-dependent processes such as breast development in males (gynecomastia).
Estrogen Receptor. Among the steroid hormone receptors, the estrogen receptor appears most susceptible to the agonistic action of xenobiotics. Estrogen receptor agonists are quite diverse in molecular structure (Figure 17.6). Several steric considerations, associated with the steroid structure, in conjunction with electrostatic (charge) properties of the outer surface of the molecule seem to dictate whether a xenobiotic can fit into the binding-pocket of the receptor and function as a receptor agonist. It is not clear why the estrogen receptor would be more susceptible to the agonistic action of xenobiotics as compared to other steroid hormone receptors. High ligand specificity may be an evolutionary trait of most “modern” receptors that is not associated with this presumed ancestral precursor to other nuclear receptor superfamily members. The estrogen receptor is often referred to as a promiscuous receptor because of this susceptibility to agonistic interactions with xenobiotics.
Some drugs are rather potent estrogens (i.e., DES); however, environmental chemicals with estrogenic activity are typically weak agonists with activity several orders-of- magnitude less than that of 17β-estradiol (Table 17.3). Because of this weak activity, xenoestrogens are typically not associated with endocrine toxicity to adult females owing to the large amount of 17β-estradiol in these individuals. However, adult males, immature individuals, and embryos all have been shown to exhibit endocrine toxicity resulting from xeno-estrogen exposure. For example, in utero exposure of male or female rodents and humans to DES causes proliferation of epithelial cells associated with the reproductive system resulting in abnormalities of this system. Gynecomastia is a common side effect of estrogenic drugs such as DES and fosfestrol when administered to adult males. The physiological consequences of xeno-estrogenic activity is typically characteristic of feminization, that is, the acquisition of female characteristics.

HO |
OH |
|
CH3 |
||
|
17b-Estradiol (endogenous hormone)
OH
O

HO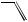
O
O
Coumestrol (botanical)
HO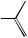
4-Nonylphenol (environmental degradation
product of some commerciallyused surfactants)
ENDOCRINE DISRUPTION |
307 |
HO
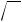 OH
OH
Diethylstillbestrol (pharmaceutical)
Cl
|
Cl |
|
|
Cl |
H |
|
|
|
|
|
|
Cl |
Cl |
|
|
o,p'-DDT |
|
|
|
|
|
|
|
|
(insecticide) |
|
|
|
Cl |
|
|
|
Cl |
|
|
Cl |
|
|
Cl |
|
|
|
|
|
|
|
Cl |
|
Cl |
|
Cl |
|
Cl |
|
|
|
|
|
|
|
Cl |
Cl |
O |
|
|
Kepone (insecticide)
Figure 17.6 Estrogen receptor agonists. Molecules are presented in a low-energy state that may represent their natural three-dimensional conformation.
Ecdysone Receptor. Ecdysteroids are a class of steroid hormones that regulate a variety of processes related to development, growth, and reproduction in insects and other arthropods but are not utilized by vertebrates. Many compounds of plant origin, or derivations thereof, have been identified that are ecdysteroid receptor agonists (i.e., cucurbitacins, withasteroids). The ecdysteroid agonists are presumed to have evolved in plants as a means of protection against insect predation. Some environmental chemicals of anthropogenic origin also have been shown to exhibit ecdysteroid receptor agonistic activity (i.e., tebufenozide) and have been exploited as insecticides due to their ability to interfere with insect development and growth.
Retinoic Acid Receptor. Most of the biological effects of retinoids are mediated through the retinoic acid receptor (RAR) and the retinoid X receptor (RXR). Both all-trans-retinoic acid and 9-cis-retinoic acid serve as agonists of RAR, while only 9-cis-retinoic acid functions as an agonist of RXR. The functional RAR exists as a heterodimer with RXR, while functional RXR exists as a homodimer. Methoprene is a juvenile hormone III analogue that mimics the activity of this insect hormone.

308 ENDOCRINE SYSTEM
Table 17.3 Potency of Some Xenoestrogens Relative to 17β-Estradiol
Chemical |
Potency |
|
|
17β-Estradiol |
100 |
Diethylstilbestrol |
74 |
4-Nonylphenol |
0.005 |
4-Octylphenol |
0.003 |
4-tert-Octylphenol |
0.00036 |
o , p -DDT |
0.00011 |
o , p -DDE |
0.00004 |
2 , 5 -Dichloro-4-biphenylol |
0.62 |
2 , 4 , 6-Trichloro-4-biphenylol |
1.0 |
2 , 3 , 4 , 5 -Tetrachloro-4-biphenylol |
0.82 |
Bisphenol A |
0.005 |
Butylbenzylphthalate |
0.0004 |
Source: Coldham, N. G. et al., 1997, Environ. Health Perspect.
105(7): 734–742.
Note: Estrogenic potency of the compounds was measured using a recombinant yeast cell bioassay.
Exposure of juvenile insects to methoprene results in various abnormalities associated with development and ultimately death. The environmental degradation product of methoprene, methoprenic acid was found to serve as an RXR agonist and specifically activate genes responsive to RXR homodimers. In addition exposure of frog larvae to methoprenic acid caused developmental deformities consistent with those that have been observed in recent years in wild populations and consistent with those caused by exposure to retinoic acid under laboratory conditions. These observations indicate that methoprenic acid functions as an RXR agonist, and that this activity could contribute to the occurrence of amphibians deformities documented in the environment.
17.3.2Hormone Receptor Antagonists
While the estrogen receptor appears somewhat unique among vertebrate nuclear hormone receptors in its promiscuity toward receptor agonists, many nuclear hormone receptors have been shown to be susceptible to chemical antagonism. Receptor antagonists are defined as chemicals that bind to a hormone receptor but do not activate the receptor. Rather, these chemicals inhibit receptor activity by preventing the endogenous hormone from binding to and activating the receptor.
Estrogen Receptor. Chemicals often bind to the estrogen receptor and function as mixed agonists/antagonists (discussed below). For example, the drug tamoxifen functions as an estrogen receptor antagonists in reproductive tissue but functions as an agonist with respect to the preservation of bone mineral density and reducing serum cholesterol concentrations. Accordingly tamoxifen can function as a prophylactic against the growth of estrogen-responsive breast cancers and osteoporosis via two different mechanisms (estrogen receptor antagonism and agonism, respectively). Other drugs that bind to the estrogen receptor as an antagonist or mixed agonist/antagonist include raloxifene,

ENDOCRINE DISRUPTION |
309 |
ICI 164,384, and toremifene. Environmental estrogen receptor antagonists include some phytochemicals (i.e., flavonoids) and PCBs (i.e., 3, 3 , 4, 4 -tetrachlorobiphenyl). Consequences of estrogen receptor antagonism are typically considered de-feminization (loss of female traits). In laboratory animal studies, estrogen receptor antagonists have been shown in females to disrupt estrous cycles, impair fertility, increase preimplantation loss, and cause embryolethality.
Androgen Receptor. Chemicals that bind to the androgen receptor in an antagonistic fashion include the pharmaceuticals spironolactone, cimetidine, cyperoterone acetate, and hydroxyflutamide. Environmental chemicals that have been shown to act as androgen receptor antagonists include the metabolites of the agricultural fungicide vinclozolin, the DDT metabolite p, p -DDE, some hydroxylated PCBs, and the organophosphate insecticide fenitrothion. The consequence of androgen receptor antagonism is typically considered demasculinization (loss of male traits). Demasculinizing effects of antiandrogens in laboratory animal studies have included reductions in the size of the ventral prostate and seminal vesicle weights along with deformities of the penis.
Glucocorticoid Receptor. Some drugs (i.e., mifepristone) elicit antagonistic activity toward the glucocorticoid receptor. This property has been associated with adverse side effects of some drugs and also has been capitalized upon therapeutically for the modulation of the glucocorticoid receptor. Antiglucocorticoids typically are steroidal compounds that are capable of binding to the receptors but are relatively ineffective in activating the receptor. As such, these compounds are typically mixed agonists/antagonists (see below). Glucocorticoid receptor antagonists can adversely affect growth, development, and other glucocorticoid-regulated processes (Table 17.1). Little is known of the ability of environmental chemicals to function as glucocorticoid receptor antagonists.
Mixed Agonists/Antagonists. Chemicals often can function as either a receptor agonist or antagonist depending on the level of endogenous hormone. A weak agonist may bind to a receptor and stimulate some low-level receptor-mediated activity in the absence of the endogenous hormone. However, in the presence of the hormone, binding of the xenobiotic to the receptor may prevent binding of the endogenous hormone, and if the xenobiotic is a much weaker activator of receptor-mediated activity, then the net effect is loss of activity. Thus, in the presence of the endogenous hormone, the xenobiotic functions as a receptor antagonist. Whether a weak xeno-agonist functions as an agonist or antagonist depends on (1) the concentration of the xeno-agonist,
(2) the binding affinity of the xeno-agonist to the receptor, (3) the concentration of the endogenous hormone to the receptor, and (4) the binding affinity of the endogenous hormone to the receptor. These compounds are classified mixed agonists/antagonists.
17.3.3Organizational versus Activational Effects of Endocrine Toxicants
Effects of receptor agonists or antagonists on endocrine related processes are often described as being either organizational or activational. An organizational effect of an endocrine toxicant is one that typically results from neonatal or prenatal exposure during which time hormones are directing various irreversible aspects of development.
- #15.08.20134.04 Mб14Hastie T., Tibshirani R., Friedman J. - The Elements of Statistical Learning Data Mining, Inference and Prediction (2002)(en).djvu
- #
- #
- #
- #
- #
- #
- #
- #15.08.201315.44 Mб23Hudlicky M, Pavlath A.E. (eds.) - Chemistry of Organic Fluorine Compounds 2[c] A critical Review (1995)(en).djvu
- #
- #
