
- •CONTENTS
- •Preface
- •Contributors
- •1 Introduction to Toxicology
- •1.1 Definition and Scope, Relationship to Other Sciences, and History
- •1.1.2 Relationship to Other Sciences
- •1.1.3 A Brief History of Toxicology
- •1.3 Sources of Toxic Compounds
- •1.3.1 Exposure Classes
- •1.3.2 Use Classes
- •1.4 Movement of Toxicants in the Environment
- •Suggested Reading
- •2.1 Introduction
- •2.2 Cell Culture Techniques
- •2.2.1 Suspension Cell Culture
- •2.2.2 Monolayer Cell Culture
- •2.2.3 Indicators of Toxicity in Cultured Cells
- •2.3 Molecular Techniques
- •2.3.1 Molecular Cloning
- •2.3.2 cDNA and Genomic Libraries
- •2.3.3 Northern and Southern Blot Analyses
- •2.3.4 Polymerase Chain Reaction (PCR)
- •2.3.5 Evaluation of Gene Expression, Regulation, and Function
- •2.4 Immunochemical Techniques
- •Suggested Reading
- •3.1 Introduction
- •3.2 General Policies Related to Analytical Laboratories
- •3.2.1 Standard Operating Procedures (SOPs)
- •3.2.2 QA/QC Manuals
- •3.2.3 Procedural Manuals
- •3.2.4 Analytical Methods Files
- •3.2.5 Laboratory Information Management System (LIMS)
- •3.3 Analytical Measurement System
- •3.3.1 Analytical Instrument Calibration
- •3.3.2 Quantitation Approaches and Techniques
- •3.4 Quality Assurance (QA) Procedures
- •3.5 Quality Control (QC) Procedures
- •3.6 Summary
- •Suggested Reading
- •4 Exposure Classes, Toxicants in Air, Water, Soil, Domestic and Occupational Settings
- •4.1 Air Pollutants
- •4.1.1 History
- •4.1.2 Types of Air Pollutants
- •4.1.3 Sources of Air Pollutants
- •4.1.4 Examples of Air Pollutants
- •4.1.5 Environmental Effects
- •4.2 Water and Soil Pollutants
- •4.2.1 Sources of Water and Soil Pollutants
- •4.2.2 Examples of Pollutants
- •4.3 Occupational Toxicants
- •4.3.1 Regulation of Exposure Levels
- •4.3.2 Routes of Exposure
- •4.3.3 Examples of Industrial Toxicants
- •Suggested Reading
- •5 Classes of Toxicants: Use Classes
- •5.1 Introduction
- •5.2 Metals
- •5.2.1 History
- •5.2.2 Common Toxic Mechanisms and Sites of Action
- •5.2.3 Lead
- •5.2.4 Mercury
- •5.2.5 Cadmium
- •5.2.6 Chromium
- •5.2.7 Arsenic
- •5.2.8 Treatment of Metal Poisoning
- •5.3 Agricultural Chemicals (Pesticides)
- •5.3.1 Introduction
- •5.3.3 Organochlorine Insecticides
- •5.3.4 Organophosphorus Insecticides
- •5.3.5 Carbamate Insecticides
- •5.3.6 Botanical Insecticides
- •5.3.7 Pyrethroid Insecticides
- •5.3.8 New Insecticide Classes
- •5.3.9 Herbicides
- •5.3.10 Fungicides
- •5.3.11 Rodenticides
- •5.3.12 Fumigants
- •5.3.13 Conclusions
- •5.4 Food Additives and Contaminants
- •5.5 Toxins
- •5.5.1 History
- •5.5.2 Microbial Toxins
- •5.5.3 Mycotoxins
- •5.5.4 Algal Toxins
- •5.5.5 Plant Toxins
- •5.5.6 Animal Toxins
- •5.6 Solvents
- •5.7 Therapeutic Drugs
- •5.8 Drugs of Abuse
- •5.9 Combustion Products
- •5.10 Cosmetics
- •Suggested Reading
- •6 Absorption and Distribution of Toxicants
- •6.1 Introduction
- •6.2 Cell Membranes
- •6.3 Mechanisms of Transport
- •6.3.1 Passive Diffusion
- •6.4 Physicochemical Properties Relevant to Diffusion
- •6.4.1 Ionization
- •6.5 Routes of Absorption
- •6.5.1 Extent of Absorption
- •6.5.2 Gastrointestinal Absorption
- •6.5.3 Dermal Absorption
- •6.5.4 Respiratory Penetration
- •6.6 Toxicant Distribution
- •6.6.1 Physicochemical Properties and Protein Binding
- •6.7 Toxicokinetics
- •Suggested Reading
- •7 Metabolism of Toxicants
- •7.1 Introduction
- •7.2 Phase I Reactions
- •7.2.4 Nonmicrosomal Oxidations
- •7.2.5 Cooxidation by Cyclooxygenases
- •7.2.6 Reduction Reactions
- •7.2.7 Hydrolysis
- •7.2.8 Epoxide Hydration
- •7.2.9 DDT Dehydrochlorinase
- •7.3 Phase II Reactions
- •7.3.1 Glucuronide Conjugation
- •7.3.2 Glucoside Conjugation
- •7.3.3 Sulfate Conjugation
- •7.3.4 Methyltransferases
- •7.3.7 Acylation
- •7.3.8 Phosphate Conjugation
- •Suggested Reading
- •8 Reactive Metabolites
- •8.1 Introduction
- •8.2 Activation Enzymes
- •8.3 Nature and Stability of Reactive Metabolites
- •8.4 Fate of Reactive Metabolites
- •8.4.1 Binding to Cellular Macromolecules
- •8.4.2 Lipid Peroxidation
- •8.4.3 Trapping and Removal: Role of Glutathione
- •8.5 Factors Affecting Toxicity of Reactive Metabolites
- •8.5.1 Levels of Activating Enzymes
- •8.5.2 Levels of Conjugating Enzymes
- •8.5.3 Levels of Cofactors or Conjugating Chemicals
- •8.6 Examples of Activating Reactions
- •8.6.1 Parathion
- •8.6.2 Vinyl Chloride
- •8.6.3 Methanol
- •8.6.5 Carbon Tetrachloride
- •8.6.8 Acetaminophen
- •8.6.9 Cycasin
- •8.7 Future Developments
- •Suggested Reading
- •9.1 Introduction
- •9.2 Nutritional Effects
- •9.2.1 Protein
- •9.2.2 Carbohydrates
- •9.2.3 Lipids
- •9.2.4 Micronutrients
- •9.2.5 Starvation and Dehydration
- •9.2.6 Nutritional Requirements in Xenobiotic Metabolism
- •9.3 Physiological Effects
- •9.3.1 Development
- •9.3.2 Gender Differences
- •9.3.3 Hormones
- •9.3.4 Pregnancy
- •9.3.5 Disease
- •9.3.6 Diurnal Rhythms
- •9.4 Comparative and Genetic Effects
- •9.4.1 Variations Among Taxonomic Groups
- •9.4.2 Selectivity
- •9.4.3 Genetic Differences
- •9.5 Chemical Effects
- •9.5.1 Inhibition
- •9.5.2 Induction
- •9.5.3 Biphasic Effects: Inhibition and Induction
- •9.6 Environmental Effects
- •9.7 General Summary and Conclusions
- •Suggested Reading
- •10 Elimination of Toxicants
- •10.1 Introduction
- •10.2 Transport
- •10.3 Renal Elimination
- •10.4 Hepatic Elimination
- •10.4.2 Active Transporters of the Bile Canaliculus
- •10.5 Respiratory Elimination
- •10.6 Conclusion
- •Suggested Reading
- •11 Acute Toxicity
- •11.1 Introduction
- •11.2 Acute Exposure and Effect
- •11.3 Dose-response Relationships
- •11.4 Nonconventional Dose-response Relationships
- •11.5 Mechanisms of Acute Toxicity
- •11.5.1 Narcosis
- •11.5.2 Acetylcholinesterase Inhibition
- •11.5.3 Ion Channel Modulators
- •11.5.4 Inhibitors of Cellular Respiration
- •Suggested Reading
- •12 Chemical Carcinogenesis
- •12.1 General Aspects of Cancer
- •12.2 Human Cancer
- •12.2.1 Causes, Incidence, and Mortality Rates of Human Cancer
- •12.2.2 Known Human Carcinogens
- •12.3 Classes of Agents Associated with Carcinogenesis
- •12.3.2 Epigenetic Agents
- •12.4 General Aspects of Chemical Carcinogenesis
- •12.5 Initiation-Promotion Model for Chemical Carcinogenesis
- •12.6 Metabolic Activation of Chemical Carcinogens and DNA Adduct Formation
- •12.7 Oncogenes
- •12.8 Tumor Suppressor Genes
- •12.8.1 Inactivation of Tumor Suppressor Genes
- •12.8.2 p53 Tumor Suppressor Gene
- •12.9 General Aspects of Mutagenicity
- •12.10 Usefulness and Limitations of Mutagenicity Assays for the Identification of Carcinogens
- •Suggested Reading
- •13 Teratogenesis
- •13.1 Introduction
- •13.2 Principles of Teratology
- •13.3 Mammalian Embryology Overview
- •13.4 Critical Periods
- •13.5 Historical Teratogens
- •13.5.1 Thalidomide
- •13.5.2 Accutane (Isotetrinoin)
- •13.5.3 Diethylstilbestrol (DES)
- •13.5.4 Alcohol
- •13.6 Testing Protocols
- •13.6.1 FDA Guidelines for Reproduction Studies for Safety Evaluation of Drugs for Human Use
- •13.6.3 Alternative Test Methods
- •13.7 Conclusions
- •Suggested Reading
- •14 Hepatotoxicity
- •14.1 Introduction
- •14.1.1 Liver Structure
- •14.1.2 Liver Function
- •14.2 Susceptibility of the Liver
- •14.3 Types of Liver Injury
- •14.3.1 Fatty Liver
- •14.3.2 Necrosis
- •14.3.3 Apoptosis
- •14.3.4 Cholestasis
- •14.3.5 Cirrhosis
- •14.3.6 Hepatitis
- •14.3.7 Oxidative Stress
- •14.3.8 Carcinogenesis
- •14.4 Mechanisms of Hepatotoxicity
- •14.5 Examples of Hepatotoxicants
- •14.5.1 Carbon Tetrachloride
- •14.5.2 Ethanol
- •14.5.3 Bromobenzene
- •14.5.4 Acetaminophen
- •14.6 Metabolic Activation of Hepatotoxicants
- •Suggested Reading
- •15 Nephrotoxicity
- •15.1 Introduction
- •15.1.1 Structure of the Renal System
- •15.1.2 Function of the Renal System
- •15.2 Susceptibility of the Renal System
- •15.3 Examples of Nephrotoxicants
- •15.3.1 Metals
- •15.3.2 Aminoglycosides
- •15.3.3 Amphotericin B
- •15.3.4 Chloroform
- •15.3.5 Hexachlorobutadiene
- •Suggested Reading
- •16 Toxicology of the Nervous System
- •16.1 Introduction
- •16.2 The Nervous system
- •16.2.1 The Neuron
- •16.2.2 Neurotransmitters and their Receptors
- •16.2.3 Glial Cells
- •16.3 Toxicant Effects on the Nervous System
- •16.3.1 Structural Effects of Toxicants on Neurons
- •16.3.2 Effects of Toxicants on Other Cells
- •16.4 Neurotoxicity Testing
- •16.4.1 In vivo Tests of Human Exposure
- •16.4.2 In vivo Tests of Animal Exposure
- •16.4.3 In vitro Neurochemical and Histopathological End Points
- •16.5 Summary
- •Suggested Reading
- •17 Endocrine System
- •17.1 Introduction
- •17.2 Endocrine System
- •17.2.1 Nuclear Receptors
- •17.3 Endocrine Disruption
- •17.3.1 Hormone Receptor Agonists
- •17.3.2 Hormone Receptor Antagonists
- •17.3.3 Organizational versus Activational Effects of Endocrine Toxicants
- •17.3.4 Inhibitors of Hormone Synthesis
- •17.3.5 Inducers of Hormone Clearance
- •17.3.6 Hormone Displacement from Binding Proteins
- •17.4 Incidents of Endocrine Toxicity
- •17.4.1 Organizational Toxicity
- •17.4.2 Activational Toxicity
- •17.4.3 Hypothyroidism
- •17.5 Conclusion
- •Suggested Reading
- •18 Respiratory Toxicity
- •18.1 Introduction
- •18.1.1 Anatomy
- •18.1.2 Cell Types
- •18.1.3 Function
- •18.2 Susceptibility of the Respiratory System
- •18.2.1 Nasal
- •18.2.2 Lung
- •18.3 Types of Toxic Response
- •18.3.1 Irritation
- •18.3.2 Cell Necrosis
- •18.3.3 Fibrosis
- •18.3.4 Emphysema
- •18.3.5 Allergic Responses
- •18.3.6 Cancer
- •18.3.7 Mediators of Toxic Responses
- •18.4 Examples of Lung Toxicants Requiring Activation
- •18.4.1 Introduction
- •18.4.2 Monocrotaline
- •18.4.3 Ipomeanol
- •18.4.4 Paraquat
- •18.5 Defense Mechanisms
- •Suggested Reading
- •19 Immunotoxicity
- •19.1 Introduction
- •19.2 The Immune System
- •19.3 Immune Suppression
- •19.4 Classification of Immune-Mediated Injury (Hypersensitivity)
- •19.5 Effects of Chemicals on Allergic Disease
- •19.5.1 Allergic Contact Dermatitis
- •19.5.2 Respiratory Allergens
- •19.5.3 Adjuvants
- •19.6 Emerging Issues: Food Allergies, Autoimmunity, and the Developing Immune System
- •Suggested Reading
- •20 Reproductive System
- •20.1 Introduction
- •20.2 Male Reproductive Physiology
- •20.3 Mechanisms and Targets of Male Reproductive Toxicants
- •20.3.1 General Mechanisms
- •20.3.2 Effects on Germ Cells
- •20.3.3 Effects on Spermatogenesis and Sperm Quality
- •20.3.4 Effects on Sexual Behavior
- •20.3.5 Effects on Endocrine Function
- •20.4 Female Reproductive Physiology
- •20.5 Mechanisms and Targets of Female Reproductive Toxicants
- •20.5.1 Tranquilizers, Narcotics, and Social Drugs
- •20.5.2 Endocrine Disruptors (EDs)
- •20.5.3 Effects on Germ Cells
- •20.5.4 Effects on the Ovaries and Uterus
- •20.5.5 Effects on Sexual Behavior
- •Suggested Reading
- •21 Toxicity Testing
- •21.1 Introduction
- •21.2 Experimental Administration of Toxicants
- •21.2.1 Introduction
- •21.2.2 Routes of Administration
- •21.3 Chemical and Physical Properties
- •21.4 Exposure and Environmental Fate
- •21.5 In vivo Tests
- •21.5.1 Acute and Subchronic Toxicity Tests
- •21.5.2 Chronic Tests
- •21.5.3 Reproductive Toxicity and Teratogenicity
- •21.5.4 Special Tests
- •21.6 In vitro and Other Short-Term Tests
- •21.6.1 Introduction
- •21.6.2 Prokaryote Mutagenicity
- •21.6.3 Eukaryote Mutagenicity
- •21.6.4 DNA Damage and Repair
- •21.6.5 Chromosome Aberrations
- •21.6.6 Mammalian Cell Transformation
- •21.6.7 General Considerations and Testing Sequences
- •21.7 Ecological Effects
- •21.7.1 Laboratory Tests
- •21.7.2 Simulated Field Tests
- •21.7.3 Field Tests
- •21.8 Risk Analysis
- •21.9 The Future of Toxicity Testing
- •Suggested Reading
- •22 Forensic and Clinical Toxicology
- •22.1 Introduction
- •22.2 Foundations of Forensic Toxicology
- •22.3 Courtroom Testimony
- •22.4.1 Documentation Practices
- •22.4.2 Considerations for Forensic Toxicological Analysis
- •22.4.3 Drug Concentrations and Distribution
- •22.5 Laboratory Analyses
- •22.5.1 Colorimetric Screening Tests
- •22.5.2 Thermal Desorption
- •22.5.6 Enzymatic Immunoassay
- •22.6 Analytical Schemes for Toxicant Detection
- •22.7 Clinical Toxicology
- •22.7.1 History Taking
- •22.7.2 Basic Operating Rules in the Treatment of Toxicosis
- •22.7.3 Approaches to Selected Toxicoses
- •Suggested Reading
- •23 Prevention of Toxicity
- •23.1 Introduction
- •23.2 Legislation and Regulation
- •23.2.1 Federal Government
- •23.2.2 State Governments
- •23.2.3 Legislation and Regulation in Other Countries
- •23.3 Prevention in Different Environments
- •23.3.1 Home
- •23.3.2 Workplace
- •23.3.3 Pollution of Air, Water, and Land
- •23.4 Education
- •Suggested Reading
- •24 Human Health Risk Assessment
- •24.1 Introduction
- •24.2 Risk Assessment Methods
- •24.2.2 Exposure Assessment
- •24.2.3 Dose Response and Risk Characterization
- •24.3 Noncancer Risk Assessment
- •24.3.1 Default Uncertainty and Modifying Factors
- •24.3.2 Derivation of Developmental Toxicant RfD
- •24.3.3 Determination of RfD and RfC of Naphthalene with the NOAEL Approach
- •24.3.4 Benchmark Dose Approach
- •24.3.5 Determination of BMD and BMDL for ETU
- •24.3.6 Quantifying Risk for Noncarcinogenic Effects: Hazard Quotient
- •24.3.7 Chemical Mixtures
- •24.4 Cancer Risk Assessment
- •24.5 PBPK Modeling
- •Suggested Reading
- •25 Analytical Methods in Toxicology
- •25.1 Introduction
- •25.2 Chemical and Physical Methods
- •25.2.1 Sampling
- •25.2.2 Experimental Studies
- •25.2.3 Forensic Studies
- •25.2.4 Sample Preparation
- •25.2.6 Spectroscopy
- •25.2.7 Other Analytical Methods
- •Suggested Reading
- •26 Basics of Environmental Toxicology
- •26.1 Introduction
- •26.2 Environmental Persistence
- •26.2.1 Abiotic Degradation
- •26.2.2 Biotic Degradation
- •26.2.3 Nondegradative Elimination Processes
- •26.3 Bioaccumulation
- •26.4 Toxicity
- •26.4.1 Acute Toxicity
- •26.4.2 Mechanisms of Acute Toxicity
- •26.4.3 Chronic Toxicity
- •26.4.5 Abiotic and Biotic Interactions
- •26.5 Conclusion
- •Suggested Reading
- •27.1 Introduction
- •27.2 Sources of Toxicants to the Environment
- •27.3 Transport Processes
- •27.3.1 Advection
- •27.3.2 Diffusion
- •27.4 Equilibrium Partitioning
- •27.5 Transformation Processes
- •27.5.1 Reversible Reactions
- •27.5.2 Irreversible Reactions
- •27.6 Environmental Fate Models
- •Suggested Reading
- •28 Environmental Risk Assessment
- •28.1 Introduction
- •28.2 Formulating the Problem
- •28.2.1 Selecting Assessment End Points
- •28.2.2 Developing Conceptual Models
- •28.2.3 Selecting Measures
- •28.3 Analyzing Exposure and Effects Information
- •28.3.1 Characterizing Exposure
- •28.3.2 Characterizing Ecological Effects
- •28.4 Characterizing Risk
- •28.4.1 Estimating Risk
- •28.4.2 Describing Risk
- •28.5 Managing Risk
- •Suggested Reading
- •29 Future Considerations for Environmental and Human Health
- •29.1 Introduction
- •29.2 Risk Management
- •29.3 Risk Assessment
- •29.4 Hazard and Exposure Assessment
- •29.5 In vivo Toxicity
- •29.6 In vitro Toxicity
- •29.7 Biochemical and Molecular Toxicology
- •29.8 Development of Selective Toxicants
- •Glossary
- •Index

IN VITRO AND OTHER SHORT-TERM TESTS |
385 |
example, in tests for cutaneous sensitization, whereas the latter is shown in impairment of the ability to resist infection.
Tests for immunotoxicity are not required by all regulatory agencies, but it is an area of great interest, both in the fundamental mechanisms of immune function and in the design of tests to measure impairment of immune function. Both of these aspects are discussed in detail in Chapter 19.
21.6IN VITRO AND OTHER SHORT-TERM TESTS
21.6.1Introduction
The toxicity tests that follow are tests conducted largely in vitro with isolated cell systems. Some are short-term tests carried out in vivo or are combinations of in vivo and in vitro systems. The latter are included because of similarities in approach, mechanism, or intent. In general, these tests measure effects on the genome or cell transformation; their importance lies in the relationship between such effects and the mechanism of chemical carcinogenesis. Mutagenicity of cells in the germ line is itself an expression of toxicity, however, and the mutant genes can be inherited and expressed in the next or subsequent generations.
The theory that the initiating step of chemical carcinogenesis is a somatic mutation is well recognized, and considerable evidence shows that mutagenic potential is correlated with carcinogenic potential. Thus the intent of much of this type of testing is to provide early warning of carcinogenic potential without the delay involved in conducting lifetime chronic feeding studies in experimental animals. Despite the numerous tests that have been devised, regulatory agencies have not yet seen it fit to substitute any of them, or any combination of them, for chronic feeding studies. Instead, they have been added as additional testing requirements. One function of such tests should be to identify those compounds with the greatest potential for toxicity and enable the amount of chronic testing to be reduced to more manageable proportions.
21.6.2Prokaryote Mutagenicity
Ames Test. The Ames test, developed by Bruce Ames and his coworkers at the University of California, Berkeley, depends on the ability of mutagenic chemicals to bring about reverse mutations in Salmonella typhimurium strains that have defects in the histidine biosynthesis pathway. These strains will not grow in the absence of histidine but can be caused to mutate back to the wild type, which can synthesize histidine and hence can grow in its absence. The postmitochondrial supernatant (S-9 fraction), obtained from homogenates of livers of rats previously treated with PCBs in order to induce certain cytochrome P450 isoforms, is also included in order to provide the activating enzymes involved in the production of the potent electrophiles often involved in the toxicity of chemicals to animals.
Bacterial tester strains have been developed that can test for either base-pair (e.g., strain TA-1531) or frameshift (e.g., strains TA-1537, TA-1538) mutations. Other, more sensitive strains such as TA-98 and TA-100 are also used, although they may be less specific with regard to the type of mutation caused.
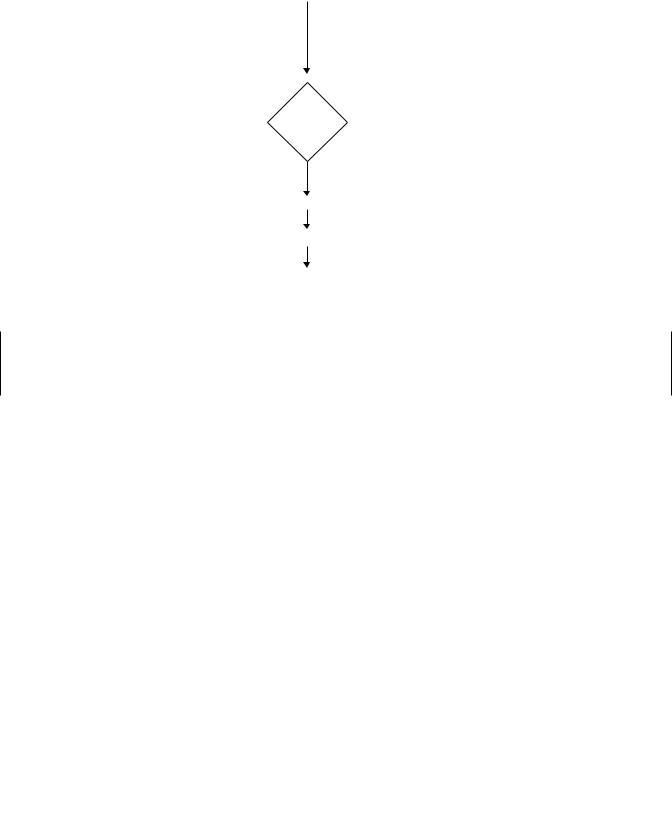
386 TOXICITY TESTING
Molten soft agar at 45°C
|
+ Test chemical in solvent |
|
or solvent alone |
|
+ Culture of S. typhimurium |
|
tester strain |
−S9 |
+ S9 (9,000 × g supernatant) |
|
fraction from PCB-induced |
|
rat liver |
Poured onto bottom agar
Incubate
Evaluate results (# of revertant colonies/plate)
Figure 21.5 Protocol for the Ames test for mutagenicity.
In brief, the test is carried out (Figure 21.5) by mixing a suspension of bacterial cells with molten top agar. This also contains cofactors, S-9 fraction, and the material to be tested. The mixture is poured onto Petri plates containing hardened minimal agar. The number of bacteria that revert and acquire the wild-type ability to grow in the absence of histidine can be estimated by counting the colonies that develop on incubation. To provide a valid test, a number of concentrations are tested, and positive controls with known mutagens are included along with negative controls that lack only the test compound. The entire test is replicated often enough to satisfy appropriate statistical tests for significance. Parallel tests without the S-9 fraction may help distinguish between chemicals with intrinsic mutagenic potential and those that require metabolic activation.
The question of correlation between mutagenicity and carcinogenicity is crucial in any consideration of the utility of this or similar tests. In general, this appears to be high, although a small proportion of both false positives and false negatives occurs. For example, certain base analogues and inorganics such as manganese are not carcinogens but are mutagens in the Ames test, whereas diethylstilbestrol (DES) is a carcinogen but not a bacterial mutagen (see Chapter 12 for additional detail).
Related Tests. Related tests include tests based on reverse mutations, as in the Ames test, as well as tests based on forward mutations. Examples include:
1.Reverse mutations in Escherichia coli. This test is similar to the Ames test and depends on reversion of tryptophane mutants, which cannot synthesize this amino acid, to the wild type, which can. The S-9 fraction from the liver of induced rats

IN VITRO AND OTHER SHORT-TERM TESTS |
387 |
can also be used as an activating system in this test. Other E. coli reverse mutation tests utilize nicotinic acid and arginine mutants.
2.Forward mutations in S. typhimurium. One such assay, dependent on the appearance of a mutation conferring resistance to 8-azaguanine in a histidine revertant strain, has been developed and is said to be as sensitive as the reverse-mutation tests
3.Forward mutations in E. coli. These mutations depend on mutation of galactose nonfermenting E. coli to galactose fermenting E. coli or the change from 5- methyltryptophane to 5-methyltryptophane resistance.
4.DNA repair. Polymerase-deficient, and thus DNA repair-deficient, E. coli has provided the basis for a test that depends on the fact that the growth of a deficient strain is inhibited more by a DNA-damaging agent than is that of a repaircompetent strain. The recombinant assay using Bacillus subtilis is conducted in much the same way because recombinant deficient strains are more sensitive to DNA-damaging agents.
21.6.3Eukaryote Mutagenicity
Mammalian Cell Mutation. The development of cell culture techniques that permit both survival and replication have led to many advances in cell biology, including the use of certain of these cell lines for detection of mutagens. Although such cells, if derived from mammals, would seem ideal for testing for toxicity toward mammals, there are several problems. Primary cells, which generally resemble those of the tissue of origin, are difficult to culture and have poor cloning ability. Because of these difficulties, certain established cell lines are usually used. These cells, such as Chinese hamster ovary cells and mouse lymphoma cells, clone readily and do not become senescent with passage through many cell generations. Unfortunately, they have little metabolic activity toward xenobiotics and thus do not readily activate toxicants. Moreover they usually show chromosome changes, such as aneuploidy (i.e., more or fewer than the usual diploid number of chromosomes).
The characteristics usually involved in these assays are resistance to 8-azaguanine or 6-thioguanine (the hypoxanthine guanine phosphoribosyl transferase or HGPRT locus), resistance to bromodeoxyuridine or triflurothymidine (the thymidine kinase or TK locus), or resistance to ouabain (the OU or Na/K-ATPase locus). HGPRT is responsible for incorporation of purines from the medium into the nucleic acid synthesis pathway. Its loss prevents uptake of normal purines and also of toxic purines such as 8-azaguanine, which would kill the cell. Thus mutation at this locus confers resistance to these toxic purine analogues. Similarly TK permits pyrimidine transport, and its loss prevents uptake of toxic pyrimidine analogues and confers resistance to them. In the absence of HGPRT or TK, the cells can grow by de novo synthesis of purines and pyrimidines. Ouabain kills cells by combining with the Na/K-ATPase. Mutation at the OU locus alters the ouabain-binding site in a way that prevents inhibition and thus confers resistance.
A typical test system is the analysis of the TK locus in mouse lymphoma cells for mutations that confer resistance to bromodeoxyuracil. The tests are conducted with and without the S-9 fraction from induced rat liver because the lymphoma cells have little activating ability. Both positive and negative controls are included, and the parameter

388 TOXICITY TESTING
measured is the number of cells formed that are capable of forming colonies in the presence of bromodeoxyuridine.
Drosophila Sex-Linked Recessive Lethal Test. The advantages of Drosophila
(fruit fly) tests are that they involve an intact eukaryotic organism with all of its interrelated organ systems and activation mechanisms but, at the same time, are fast, relatively easy to perform, and do not involve mammals as test animals. The most obvious disadvantages are that the hormonal and immune systems of insects are significantly different from those of mammals and that the nature, specificity, and inducibility of the cytochrome P450s are not as well understood in insects as they are in mammals.
In a typical test, males that are 2 days postpuparium and that were raised from eggs laid within a short time period (usually 24 hours) are treated with the test compound in water to which sucrose has been added to increase palatability. Males from a strain carrying a gene for yellow body on the X chromosome are used. Preliminary tests determine that the number of offspring of the survivors of the treatment doses (usually 0.25 LD50 and 0.5 LD50) are adequate for future crosses. Appropriate controls, including a solvent control (with emulsifier if one was necessary to prepare the test solution), and a positive control, such as ethyl methane sulfonate, are routinely included with each test. Individual crosses of each surviving treated male with a series of three females are made on a 0- to 2-, 3- to 5-, and 6- to 8-day schedule. The progeny of each female is reared separately, and the males and females of the F1 generation are mated in brother-sister matings. If there are no males with yellow bodies in a particular set of progeny, it should be assumed that a lethal mutation was present on the treated X chromosomes. A comparison of the F2 progeny derived from females inseminated by males at different times after treatment allows a distinction to be made between effects on spermatozoa, spermatids, and spermatocytes.
In the Basc (Muller-5) test shown in Figure 21.6, the strain used for the females in the F1 cross is a multiple-marked strain that carries a dominant gene for bar eyes and recessive genes for apricot eyes and a reduction of bristles on the thorax (scute gene). (Basc is an acronym for bar, apricot, and scute.)
Related Tests. Many tests related to the two types of eukaryote-mutation tests are discussed earlier in this section, and many of them are simply variations of the tests described. Two distinct classes are worthy of mention: the first uses yeasts as the test organisms, and the second is the spot test for mutations in mice.
One group of yeast tests includes tests for gene mutations and strains that can be used to detect forward mutations in genes that code for enzymes in the purine biosynthetic pathway; other strains can be used to detect reversions. Yeasts can also be used to test for recombinant events such as reciprocal mitotic recombination (mitotic crossing over) and nonreciprocal mitotic recombination. Saccharomyces cerevisiae is the preferred organism in almost all these tests. Although they possess cytochrome P450s capable of metabolizing xenobiotics, their specificity and sensitivity are limited as compared with those of mammals, and an S-9 fraction is often included, as in the Ames test, to enhance activation.
The gene mutation test systems in mice include the specific locus test, in which wildtype treated males are crossed with females carrying recessive mutations for visible phenotypic effects. The F1 progeny have the same phenotype as the wild-type parent unless a mutation, corresponding to a recessive mutant marker, has occurred. Such tests
- #15.08.20134.04 Mб17Hastie T., Tibshirani R., Friedman J. - The Elements of Statistical Learning Data Mining, Inference and Prediction (2002)(en).djvu
- #
- #
- #
- #
- #
- #
- #
- #15.08.201315.44 Mб26Hudlicky M, Pavlath A.E. (eds.) - Chemistry of Organic Fluorine Compounds 2[c] A critical Review (1995)(en).djvu
- #
- #
