
Micro-Nano Technology for Genomics and Proteomics BioMEMs - Ozkan
.pdf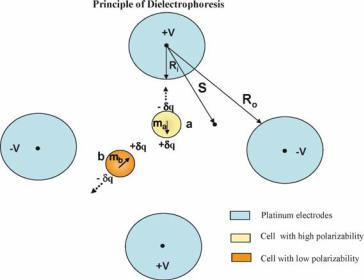
62 |
MIHRIMAH OZKAN ET AL. |
3.15. BASIS OF DIELECTROPHORESIS
The basic dielectrophoretic effect is demonstrated in figure 3.7, in which electrodes of spherical geometry are used to generate an inhomogeneous (non uniform) electric field. Many different electrode geometries have been used in DEP applications, but the spherical type is used generally as a model system because the non uniform field it generates can be described by a simple formula. The dipole moment m induced in a particle can be represented by the generation of equal and opposite charges +q and −q at the particle boundary. The magnitude of the induced charge q is small and is equivalent to 0.1% of the net surface charge normally carried by the cells and can be generated within a micro second [104]. If the applied electric field is non-uniform as shown in figure 3.7, the local electric field E1 and the resulting force (E1δq) on each side of the particle will be different. Thus, depending on the relative polarizability of the particle with respect to the surrounding medium, it will be induced to move towards the inner electrode (from figure 3.7) or the region of high electric field (Positive DEP), or towards the outer electrode (from figure 3.7) where the field is weaker (negative DEP).
Following established theory [101] the DEP force FDEP acting on a spherical particle of radius r suspended in a fluid of absolute dielectric permittivity εm is given by equation 3.1
FDEP = 2πr 3εm α E 2 |
(3.1) |
where α is a parameter defining the effective polarizability of the particle and the factor
FIGURE 3.7. Principle of generation of positive and negative dielectrophoretic traps. The dielectrophoretic force is dependent on the polarizability of the particles and the surrounding medium and the applied root mean square voltage. Particle a experiences positive dielectrophoretic force and is trapped over the electrode. Particle b experiences negative dielectrophoretic force and is trapped in regions between the electrodes [102].
MICROARRAY AND FLUIDIC CHIP FOR EXTRACELLULAR SENSING |
63 |
E 2 is proportional to the gradient of the applied electric field. The polarizability parameter α varies as a function of the frequency of the applied electric field and depending on the dielectric properties of the particle and the surrounding medium can theoretically have the values between +1.5 and −0.5. The value of α at frequencies below 1 kHz is determined largely by the polarizations associated with the particle surface charge, while with increasing frequency first the effective conductivity, and then the effective dielectric permittivity become the dominant contributing factors. A positive value of α leads to an induce dipole moment aligned in the direction of the field as shown for particle a in figure 3.7 and to a positive DEP force. A negative α results in an induced dipole moment aligned against the field as for particle b and produces negative DEP. The fact that the field appears as E 2 in equation 3.1 also indicates that reversing the polarity of the applied voltage does not reverse the DEP force. AC voltages can be employed for a wide range of applied frequencies (typically varying from 500 Hz to 50 MHz), the dielectric properties of the particle are encompassed in the parameter α and can be fully exploited. Equation 3.1 represents the conventional dipole approximation of DEP, and is valid providing the field is not too inhomogeneous as can occur very close to the electrode edges. Also with some other electrode geometries, regions of zero electric field are generated, in which the net dipole moment induced must also therefore be zero. In such a case a general multipolar theory must be used to describe the DEP force accurately [61].
3.16. MICROELECTRODES AND DIELECTROPHORESIS
In the early stages of the use of dielectrophoresis, the electrodes took the form of metal sheets, wires, rods and pins [107]. The electrodes were considerably larger than the bioparticles under study. With the advent of microelectrodes to this field of study [Jones et al., 1995, 32, 81, 112], using photolithography and associated semiconductor microfabrication technologies, dielectrophoresis has been easily integrated for cell separation and study applications essentially suited for “lab-on-a-chip” devices (Ward, 1997).
By reducing the electrode dimension it can be demonstrated based on the mathematical representation of the dielectrophoretic force that the values of the electric field strength andE 2 at the particle location increase as the electrode scale is reduced while maintaining the applied voltage. Reducing the radius of curvature by 100 fold produces a 1000 fold increase in E 2. This can be explained by using dimensional analysis. The factor E 2 has units V2m−3, thus from dimensional analysis, an n fold change in the electrode scale, for any electrode design, results in an operating voltage change of n3/2. So for a 100 fold reduction in electrode size, a 1000 fold reduction in the operating voltage will produce the same DEP force on the particle in the same relative location. In addition to the advantage if using lower operating voltages for a desired DEP force there is also a significant reduction in the electrical heating and electrochemical effects. The energy deposition from the field is proportional to σE2, where σ is the conductivity of the suspending fluid. Also as the size of the electrodes decreases the surface area of the electrodes in contact with the fluid is decreases, surface electrochemical process is also reduced. As particle radius is reduced below 0.1 µm, the values for E 2 greater than 1017 V2 m−3 are required for the DEP force to be ten times larger than the Brownian force. If the conductivity of the suspending medium is larger than around 0.1Sm−1, such high field strengths have the capability of creating hydrodynamic
64 |
MIHRIMAH OZKAN ET AL. |
effects as a result of localized heating of the fluid. Sub micron scale electrodes have been used to achieve stable DEP levitation and trapping of particles as small as 14nm, but thermal gradients and hydrodynamic effects were reported to influence this effect significantly [89]. Also, microelectrodes used for DEP separation of bioparticles often have a periodically repeating interdigitated geometry. The value of E 2 decays exponentially with distance from the electrode plane. The decay rate is inversely proportional to the characteristic electrode periodicity.
3.17. DIELECTRIC PROPERTIES OF CELLS
Biological cells exhibit large induced dipole moments that are highly dependent on the frequency of the applied field [101, 102]. This is caused by the fact that cells consist of adjacent structures of materials that have very different electrical properties, leading to large frequency-dependent interfacial polarizations at the boundaries between structures. The cell membrane consists of the lipid bilayer that contains many protein structures. This is not only thin but highly insulating. The inside of the cell is complex and can contain not only membrane-covered particulates such as mitochondria, vacuoles and a nucleus, but also many dissolved charged particles. Thus, whilst the conductivity of the membrane tends to be around 10−7 S m−1, that of the interior is around 1 S m−1, a difference of around 107. On cell death the membrane becomes more permeable and its conductivity increases by a factor of 104, with the cell contents freely exchanging material with the external medium. These large changes in the dielectric properties on cell death shows up as a large change in the dielectric polarizability. Small changes in membrane properties can also be detected using DEP and has been used for selective separation of live or dead cells. As cells of different species, stages of differentiation or physiological state can have different morphology, structure and composition, this results in differences in the in polarizability and finally in the DEP response.
3.18. EFFECT OF ELECTRIC FIELDS ON CELLS
When a cell is exposed to an electric field, it becomes electrically polarized. At low frequencies, the cell interior is shielded from the applied field by the effective capacitance of cytoplasmic membrane, and as a result the membrane sustains the full electric potential applied to the cell [11]. The maximum value of the induced membrane potential occurs at the two ‘poles’ of the cell, in a radial direction parallel to the field, and has a value of about 1.5 rE. Where r is the radius of the particle. If this induced membrane potential exceeds a value of around 1V, the electrical stress on the membrane can be sufficient to facilitate the useful effects of electroporation and electrifusion of cells [92]. For cells with radius of around 2.5 to 5 µm, the applied field required to produce such effects is 1 − 3 × 105 Vm−1. As already described, a value for the factor E 2 of around 1013 V2m−3 is required to produce significant DEP force, and by calculations it is determined that this can be achieved with an applied field of less than 104 Vm−1.The dielectrophoretic manipulation of cells should therefore not lead to any irreversible damage to the cells, and this has been confirmed in practice [34, 79].

MICROARRAY AND FLUIDIC CHIP FOR EXTRACELLULAR SENSING |
65 |
In experiments to demonstrate the DEP separation of viable and unviable yeast, the subsequent viability of the cells was checked by staining with methylene blue and plate counts [79]. The viabilities of erythrocytes dielectrophoretically separated from leukemia cells have also been confirmed with trypan blue dye (Becker et al., 1997), and CD34+ cells have been successfully cultured following DEP separation [127]. Fuhr et al. have also demonstrated that fibroblasts can be successfully cultivated without any significant changes to their viability, motility, anchorage, cell-cycle time, when exposed continuously over a period of 3 days to fields generate by types of microelectrodes used in DEP [34]. Even bacteria have shown no significant changes o their physiological characteristics after long exposures to DEP forces [79].
Considering the effect of DEP and the use of microfabrication technology, it is possible to integrate the two techniques to develop electrical sensors using mammalian cells as both the sensing as well as the transduction elements. To develop the technique of electrical sensing it first becomes essential to determine the parameters of DEP that are essential to isolate and position the individual cell types and then expose them to the chemical agents under test. The detection limit for a specific chemical associated with a specific cell type can be determined using this technique. The variation in the extracellular electrical signal is analyzed using fast fourier transformation (FFT) techniques that yield identification tags also known as “Signature Patterns”. Finally the veracity of the technique is verified using conventional fluorescent chemistry methods.
3.19. CELL TYPES AND THE PARAMETERS FOR DIELECTROPHORETIC PATTERNING
In our experiments we have used two types of mammalian cells that have electrically excitable cell membranes. The first are the rat hippocampal cells obtained from the H19-7 cell line from ATCC. The second is the primary rat osteoblast culture. The parameters for DEP isolation and positioning are determined in each case and are summed up in table 3.1. A gradient AC field is set up among the electrodes by adjusting the parameters of the applied frequency, peak-to-peak voltage and conductivity of the separation buffer. Cells under the absence of an electric field have a uniformly distributed negative charge along the membrane surface. On applying the gradient AC field a dipole is induced based on the cell’s
TABLE 3.1. Parameters for positive and negative DEP for neurons and osteoblasts
|
Separation |
Conductivity of |
Positive DEP |
Negative DEP |
Cross over |
Vpp |
Cell Type |
buffer for DEP |
buffer solution (m S/cm) |
frequency |
frequency |
frequency |
(Volts) |
|
|
|
|
|
|
|
Neurons |
250 m M Sucrose/ |
1.2 |
4.6 MHz |
300 kHz |
500 kHz |
8 |
|
1640 RPMI |
|
|
|
|
|
Osteoblasts |
250m M |
6.07 |
1.2 MHz |
75 kHz |
120 kHz |
2 |
|
Sucrose/ |
|
|
|
|
|
|
Dubecco’s |
|
|
|
|
|
|
modified Eagele |
|
|
|
|
|
|
Medium |
|
|
|
|
|
|
(DMEM) |
|
|
|
|
|
|
|
|
|
|
|
|

66 |
MIHRIMAH OZKAN ET AL. |
FIGURE 3.8. Schematic diagram of the integrated recording/measurement system. Single cell arrays are formed over the platinum electrodes/ sensing sites by setting up positive dielectrophoretic traps over the sensing sites. Gradient electric fields are set up over the sensing platform using a function generator. The chemical analyte mixture is circulated over the sensing platform via the microfluidic chamber using a pumping system. Electrical monitoring of the sensing sites is achieved by connecting micromanipulator probes to the electrode leads emerging from each sensing site. The extracellular electrical activity is measured with respect to a reference electrode denoted by R. The electrical activity is monitored using an oscilloscope. The activity is recorded using LabVIEW. Simultaneous optical monitoring is achieved through a CCD camera [111].
dielectric properties and due to the non uniform electric field distribution the electrically excitable cells experience a positive dielectrophoretic force that causes their migration to the electrodes, which are the regions of high electric fields [110]. In this manner neurons and osteoblasts are isolated and positioned over electrodes.
3.20. BIOSENSING SYSTEM
The biosensing system comprises of a chip assembly and an environmental chamber to maintain a stable local environment for accurate data acquisition. The biosensing system is schematically represented in figure 3.8.
3.21. CHIP ASSEMBLY
The microelectrode array fabrication and cell patterning has been achieved using a previous procedure [110] and is now briefly described. A 5 × 5 microelectrode array comprising of platinum electrodes (diameter: 80µm, center-to-center spacing: 200µm) spanning a surface area of 0.88 × 0.88mm2 on a silicon/silicon nitride substrate with electrode leads (6 µm thick) terminating at electrode pads (100 µm × 120 µm) is fabricated using standard
MICROARRAY AND FLUIDIC CHIP FOR EXTRACELLULAR SENSING |
67 |
lithography techniques. To achieve a stable local microenvironment the microelectrode array is integrated with a silicone chamber (16mm × 16mm × 2.5mm) with a microfluidic channel (50 µm, wide), to pump in the testing agent and pump out the test buffer once the sensing process has been completed. The flow rate of the buffer is 40µL/min. The silicone chamber is provided with an opening (8mm × 8mm × 2.5mm) and is covered by a glass cover slip for in-situ monitoring. Simultaneous electrical and optical monitoring is achieved by using a MicrozoomTM(Nyoptics Inc, Danville, CA) optical probe station under 8× and 25× magnification. The Electrical stimulation and measurements are achieved utilizing micromanipulators (Signatone, Gilroy, CA).
3.22. ENVIRONMENTAL CHAMBER
The optical probe station along with the chip assembly is enclosed by an acrylic chamber (S&W Plastics, Riverside, CA). The environment in the chamber is controlled so as to maintain a constant temperature of 37◦C. A heat gun (McMaster, Santa Fe Springs, CA) inside the chamber heats the air in the chamber and this is linked to a temperature controller (Cole Parmer, Vernon Hills, Illinois) that stops the heat gun from functioning above the desired temperature. A 6” fan (McMaster, Santa Fe Springs, CA) inside the chamber circulates the hot air to maintain temperature uniformity throughout the chamber and is monitored by a J-type thermocouple probe attached to the temperature controller. The carbon dioxide concentration inside the chamber is maintained at 5% and is humidified to prevent excessive evaporation of the medium. This chamber with all of its components will ensure cell viability over long periods of time and stable cell physiology in the absence of the chemical agents.
3.23. EXPERIMENTAL MEASUREMENT SYSTEM
The measurement system comprises of extracellular positioning, stimulating and recording units. The cells were isolated and positioned over single electrodes by setting up a gradient AC field using an extracellular positioning system comprising of a pulse generator (HP 33120A) and micromanipulators (Signatone, Gilroy, CA). The signal from the pulse generator was fed to the electrode pads of the selected electrodes using the micromanipulators. The extracellular recordings from the individual osteoblasts obtained from the electrode pads were amplified and recorded on an oscilloscope (HP 54600B, 100 MHz). The supply and measurement systems are integrated using general purpose interface bus (GPIB) control and controlled through LabVIEW (National Instruments, Austin, TX).
3.24. CELL CULTURE
3.24.1. Neuron Culture
The H19-7 cell line is derived from hippocampi dissected from embryonic day 17 (E17) Holtzman rat embryos and immortalized by retroviral transduction of temperature sensitive tsA58 SV40 large T antigen. H19-7 cells grow at the permissive temperature
68 |
MIHRIMAH OZKAN ET AL. |
(34◦C) in epidermal growth factor or serum. They differentiate to a neuronal phenotype at the nonpermissive temperature (39C) when induced by basic fibroblast growth factor (bFGF) in N2 medium (DMEM-high glucose medium with supplements). H19-7/IGF-IR cells are established by infecting H19-7 cells with a retroviral vector expressing the human type I insulin-like growth factor receptor (IGF-IR). The cells are selected in medium containing puromycin.H19-7/IGF-IR cells express the IGF-IR protein. IGF-IR is known to send two seemingly contradictory signals inducing either cell proliferation or cell differentiation, depending on cell type and/or conditions. At 39◦C, expression of the human IGF-IR in H19-7 cells induces an insulin-like growth factor (IGF) I dependent differentiation. The cells extend neuritis and show increased expression of NF68. This cell line does not express detectable levels of the SV40 T antigen.Following spin at 100 × g for 10 minutes at room temperature; cells were re-suspended in a separation buffer (see Table 3.1). The density of the re-suspended cells (2500 cell/mL) ensured single cell positioning over individual electrodes. Separation buffer used for neurons contained 250 mM sucrose/1640 RPMI (Roswell Park Memorial Institute), with a conductivity of 1.2 mS/cm and a pH of 7.48. The separation buffer was replaced by a buffer comprising of minimum essential medium/10% Fetal Bovine Serum (FBS)/5% Phosphate buffer saline (PBS) of conductivity 2.48 mS/cm and pH of 7.4 suitable for cell viability.
3.24.2. Primary Osteoblast Culture
Primary rat osteoblast cells are cultured to a concentration of 2,500 cells in 1mL for sensing experiments. To achieve the patterning of a single cell over a single electrode, a 10 µL of cell culture solution was mixed with 500 µL Dulbeco modified eagle medium (DMEM; Gibco, Grand Island NY) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island NY), 100µg/mL penicillin, and 100gµg/mL streptomycin (P/S; Gibco, Grand Island NY). The cells were centrifuged and re-suspended in 1mL of separation buffer consisting of 1:9 dilutions of Phosphate Buffer Saline/250mM Sucrose (Sigma, St Louis) and de-ionized water (w/v). The conductivity of the separation buffer was 4.09mS/cm and with a pH of 7.5. The separation buffer is replaced with a test buffer ((DMEM)/ Fetal Bovine Serum (FBS)/Phosphate Buffer Saline (PBS)) with conductivity of 2.5 mS/cm and a pH of 7.4.
3.25. SIGNAL PROCESSING
Changes in the extracellular potential shape have been used to monitor the cellular response to the action of environmental agents and toxins. The extracellular electrical activities of a single osteoblast cell are recorded both in the presence and absence of chemical agents and the modulation in the electrical activity is determined. However, the complexity of this signal makes interpretation of the cellular response to a specific chemical agent difficult to interpret. It is essential to characterize the signal both in time domain and frequency domain for extracting the relevant functional information.
The use of power spectral density analysis as a tool for classifying the action of a chemically active agent was investigated and found to offer a suitable technique for data analysis. The power of the extracellular potential is a better indicator of general shape than the peak-to-peak amplitude monitored in this work.
MICROARRAY AND FLUIDIC CHIP FOR EXTRACELLULAR SENSING |
69 |
Spatial and temporal modifications to the extracellular potential have been used to monitor the cellular response to the action of chemical and biological analytes. The extracellular electrical activity from single neurons and osteoblasts are recorded from the sensing sites both in the presence and absence of the chemical analytes under study. The modulation in the electrical activity is then determined in each case. However, the complexity of this signal makes interpretation of the cellular physiological response to a specific chemical analyte difficult to interpret. Hence there arises a requirement for signal characterization in both the time domain and frequency domain for extracting the pertinent functional information. Power spectral density analysis of the acquired data was found to be a suitable and reliable tool for data analysis. The power of the extracellular potential is a better indicator of general shape and variations due to the effect of the chemical analyte than the monitored peak-to-peak amplitude of the signal. This is achieved by examining the RMS power in different frequency bands.
Using Fast Fourier Transformation (FFT) analysis, the shifts in the signal’s power spectrum are analyzed. The FFT analysis indicates the modulation in the frequency of the extracellular potential burst rate and hence is termed as “frequency modulation” and generates the signature pattern vector (SP) (Yang et al., 2003). This SP is unique to the chemical analyte and the cell type.
3.26. SELECTION OF CHEMICAL AGENTS
To obtain the effect of a broad spectrum of chemical agents ranging from highly toxic and physiologically damaging to relatively less toxic to determine and evaluate the time window of response of a particular cell type for a specific known agent based on varying concentrations and finally determines the limit of detection for a specific chemical agent. All the experiments were conducted based on the hypothesis that a unique SP would be generated for each cell type for a specific chemical. This was hypothesized as it has been scientifically proven that different chemicals bind to different ion channel receptors thus, modifying the electrical response of the cell in a unique manner. We present here the responses of single excitable cells to the effect of the following chemical agents: ethanol, hydrogen peroxide, ethylene diamene tetra acetic acid (EDTA), and pyrethroid.
3.26.1. Ethanol
Ethanol produces anesthetic effects but in a milder form as compared to pentobarbitone and ketamine, though the mechanism of action is essentially assumed to be the same [123]. We hypothesized that determination of single cell ethanol sensitivity would help us identify the lowest threshold concentration, for the family of chemicals whose physiological response mechanism would mimic that of ethanol.
3.26.2. Hydrogen Peroxide
It is one of the major metabolically active oxidants present in the body and leads to apoptosis. Hydrogen peroxide also leads to the degradation of cells. As the behavior of hydrogen peroxide in-vivo is similar to the behavioral responses obtained from exposure
70 |
MIHRIMAH OZKAN ET AL. |
to carcinogenic chemicals like rotenone, we estimated that hydrogen peroxide would make an ideal candidate for sensing studies [39].
3.26.3. Pyrethroid
They are active ingredients in most of the commercially used pesticides. The pyrethroid share similar modes of action, resembling that of DDT. Pyrethroid is expected to produce a “knock down” effect in-vivo; the exact in-vitro response at a cellular level has not yet been understood. Hence they are ideal candidates for the analysis of this genre of chemicals.
3.26.4. Ethylene Diamene Tetra Acetic Acid (EDTA)
EDTA belongs to a class of synthetic, phosphate-alternative compounds that are not readily biodegradable and once introduced into the general environment can re-dissolve toxic heavy metals. Target specificity of EDTA in a single electrically excitable cell has not been electrically analyzed to date.
3.27. CHEMICAL AGENT SENSING
3.27.1. Signature Pattern for Control Experiments
In order to determine the signature pattern vector corresponding to a specific chemical, the initial activity pattern vector for each cell type was determined. Using the process of Dielectrophoresis, a single cell was positioned over a single electrode and its initial electrical activity was recorded. As the first stage, control experiments were performed in which single osteoblasts and neurons were exposed to the sensing buffer in the absence of chemical agents, and the extracellular signal was recorded and analyzed to generate the initial (background) SP pertaining to osteoblast’s and neuron’s characteristic burst rate depending on its physiological condition. FFT analysis extracted the characteristic burst frequency from the firing pattern. The characteristic burst frequency was determined to be at 668 Hz for a single osteoblast and 626 Hz for a single neuron. This corresponds with neuronal electrical activity determined from other topographical methods [63, 76].
3.28. ELECTRICAL SENSING CYCLE
Chemical agents are first premixed individually with the sensing buffer and introduced into the sensor system. The modified electrical activity due to presence of chemical agents was recorded. Testing of a specific chemical agent was performed in a cyclic manner with each cycle comprising of three phases. The time duration of each phase was on an average of 60 seconds. The data presented here is averaged over fifteen cycles (n = 15). The action of each chemical agent at decrementing concentration ranges (step size in the higher concentration range: 500 ppm, lower concentration range (<1000 ppm): 50 ppm) was determined by monitoring the electrical activity at 5 seconds intervals for the first 30 seconds and then at 30 seconds intervals over a period of 180 seconds. This constitutes a single sensing cycle. In the
MICROARRAY AND FLUIDIC CHIP FOR EXTRACELLULAR SENSING |
71 |
presence of each specific chemical agent (ethanol concentrations ranging from 5000 ppm to 5 ppm for neurons and 5000 ppm to 15 ppm for osteoblasts, hydrogen peroxide: 5000 ppm to 10 ppm for neurons and 5000 ppm to 20 ppm for osteoblasts, pyrethroid: 5000 ppm to 250 ppb for neurons and 5000 ppm to 850 ppb for osteoblasts, EDTA: 5000 ppm to 150 ppm for neurons and 5000 ppm to 250 ppm for osteoblasts), pronounced modifications in the extracellular action potentials were observed. The detection limits for a single neuron was—ethanol: 9 ppm, hydrogen peroxide: 19 ppm, pyrethroid: 280 ppb, and EDTA: 180 ppm. Similarly the detection limits for a single osteoblast wasethanol: 19 ppm, hydrogen peroxide: 25 ppm, pyrethroid: 890 ppb and EDTA: 280 ppm. The lowest single neuron sensitivity as estimated theoretically by the existing methods of averaging and iteration indicate the lowest concentrations determined that are—ethanol (MW = 46.07): 25 ppm (Maldve et al., 2002) as compared to the experimentally obtained detection limit of 9ppm hydrogen peroxide (MW = 34.01): 45 ppm as compared to 19 ppm [9], pyrethroid (MW = 38.3): 550 ppb as compared to 280 ppb [144], EDTA (MW = 292.2): 300 ppm to 180 M [130].
3.29. ETHANOL SENSING
3.29.1. Single Neuron Sensing
The initial concentration of ethanol used was 5000ppm and the modified electrical activity was recorded. The concentration of ethanol was decremented in a stepwise manner and in each case the modified electrical activity was recorded. The lowest concentration of ethanol sensed by a single neuron was 9ppm.The SP corresponding to the response of a single neuron to a specific agent in the frequency domain is represented in figure 3.9(A). The obtained SP is unique to a specific chemical agent and remains unchanged for varying concentrations of the specific agent. The SP obtained from a single neuron in the absence of a chemical agent indicates the initial control characteristic burst rate of 626 Hz. Addition of ethanol leads to its binding to M1 and M2 regions on the outside face of the GABAA and Glycine receptor gated Cl− ion channels [77]. This increases the duration of the channel openings causing a strong inhibitory ionic current associated with Cl− influx and decreased the frequency of firing to 314 Hz (Fig 3.9(A)).
3.29.2. Single Osteoblast Sensing
Single osteoblast cells were positioned over individual electrodes. The sensing agent was then introduced onto the microelectrode array using the microfluidic inlet channel. The initial concentration of ethanol used was 5000ppm and the modified electrical activity was recorded. The concentration of ethanol was decremented in a stepwise manner and in each case the modified electrical activity was recorded. The lowest concentration of ethanol sensed by a single osteoblast was 19 ppm. FFT analysis was performed on the acquired data pertaining to the modified extracellular potential to yield the SP. The instant at which the chemical is added to the chip system is denoted by t = 0sec. Figure 3.9(B) represents the SP for a single osteoblast due to the action of ethanol at 19ppm. Osteoblasts have an unmodulated firing rate of 668Hz. This corresponds to the frequency of firing of osteoblast in the absence of a chemical agent. There are two eigen vectors (514Hz, and 722Hz) in the
