
Micro-Nano Technology for Genomics and Proteomics BioMEMs - Ozkan
.pdf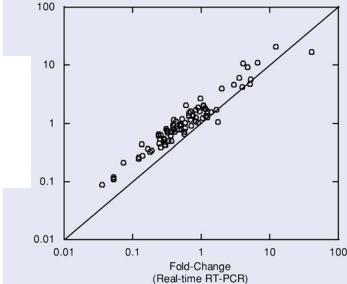
42 |
DOMINICK SINICROPI, MAUREEN CRONIN AND MEI-LAN LIU |
Fold-Change (Agilent cDNA Microarray)
FIGURE 2.8. Comparison of gene expression profiles determined by cDNA microarrays and TaqMan RT-PCR.
RT-PCR, although the degree of bias for 47 individual transcripts was not predictable [46]. The Spearman rank correlation coefficient (our calculation from the supplemental data supplied by Yuen and coworkers) between expression fold-changes for all 47 genes measured by cDNA arrays and real time RT-PCR was 0.725.
Results obtained in our laboratory comparing cDNA microarray and TaqMan expression profiles were in general agreement with the studies cited above. The two samples we analyzed were different mixtures of RNA from various human tissues that we refer to as Human Reference RNA A and B. The relative expression ratio of 91 genes in the samples was determined by TaqMan RT-PCR and was compared with the expression ratio determined by Agilent cDNA microarrays as shown in Figure 2.8. Although the agreement for individual transcripts can vary quantitatively, an excellent correlation was observed between the rank orders of expression profiles determined by these two technology platforms (Spearman’s R = 0.943). Similar to the results of Yuen and coworkers, we observed a systematic bias in the magnitude of expression ratios measured by microarrays as compared with RT-PCR. Overall, our results provide further support for the qualitative validity of expression differences measured by cDNA microarrays.
2.4.2. Comparison of Oligonucleotide Arrays with Other Gene Expression Profiling Methods
Published studies report varying degrees of correlation between expression profiles determined by oligonucleotide arrays and RT-PCR. Yuen and coworkers reported qualitatively similar expression profiles using Affymetrix GeneChip arrays and real time RT-PCR. However, as with cDNA microarrays, the magnitude of expression differences measured by
GENE EXPRESSION PROFILING UTILIZING MICROARRAY TECHNOLOGY AND RT-PCR |
43 |
oligonucleotide microarrays tended to underestimate the expression differences measured by real time RT-PCR [46]. The Spearman rank correlation coefficient (our calculation from the supplemental data supplied by Yuen and coworkers) between expression fold-changes for all 47 genes measured by Affymetrix arrays and real time RT-PCR was 0.683. As noted above for cDNA arrays, the degree of bias varied for individual genes. In another study, Van den Boom and coworkers [47] studied the expression profiles of different grades of astrocytomas using Affymetrix microarrays and compared their results for 12 genes determined by real-time RT-PCR. Eight pairs of gliomas were profiled by both methods. Correlation coefficients calculated for each of the 12 genes were in the range of 0.48–0.98. In contrast, Baum and coworkers measured the expression profile of 56 genes induced by osmotic shock in yeast [29] using a different in situ synthesized oligonucleotide microarray. They observed a Spearman rank correlation coefficient of 0.972 when their microarray results were compared with the expression profile for the same samples determined by real-time RT-PCR.
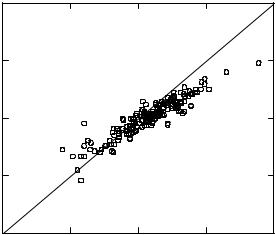
In our laboratory, we compared the expression profiles of the Human Reference RNA samples mentioned above as determined using Affymetrix microarrays and TaqMan realtime RT-PCR. Prior to hybridization, the RNA samples were carried through two cycles of amplification before labeling the final aRNA product. The original RNA samples were evaluated by RT-PCR for 180 genes. Comparison of the expression differences determined by the two methods produced a Spearman rank correlation coefficient of 0.911 although disagreement between the methods was observed for several individual genes as seen in Figure 2.9. In addition, the fold-change measured by microarrays was biased towards underestimation of the fold-change measured by real time RT-PCR.
Fold-Change (Affymetrix Microarray)
100 |
|
|
|
|
10 |
|
|
|
|
1 |
|
|
|
|
0.1 |
|
|
|
|
0.01 |
|
|
|
|
0.01 |
0.1 |
1 |
10 |
100 |
Fold-Change
(Real-time RT-PCR)
FIGURE 2.9. Comparison of gene expression profiles determined by Affymetrix oligonucleotide microarrays and TaqMan RT-PCR.
44 |
DOMINICK SINICROPI, MAUREEN CRONIN AND MEI-LAN LIU |
2.4.3. Comparison of cDNA and Oligonucleotide Microarray Expression Profiles
Recently, Lee and coworkers [48] proposed a method for validation of thousands of gene expression levels at a time by comparing results obtained with cDNA and oligonucleotide microarrays that are subject to different artifacts. In their study, several thousand transcripts were profiled in 60 human cancer cell lines (the NCI-60 panel) using both cDNA and Affymetrix GeneChip microarrays. The investigators posit that agreement of results obtained for a single transcript across many samples provides support for the validity of data obtained using both technology platforms. On the other hand, disagreement for an individual transcript does not indicate which (or if either) of the technology platforms generated a valid result. The correlation coefficients calculated for 2,344 Unigene-matched transcripts on the two microarray platforms were broadly distributed between −0.5 and 1.0. A consensus set of transcripts was identified that produced similar expression profiles on both cDNA and Affymetrix GeneChip microarrays. The observation by Lee and coworkers that expression profiles generated by cDNA and oligonucleotide microarrays are discordant for many transcripts supports the view that inaccuracies can arise from cross-hybridization, sequence variability of hybridization efficiency, as well as variability in the design, synthesis, manufacture of probes and target labeling. Other investigators have reached similar conclusions based on comparisons of expression profiles for the same samples generated on different microarray platforms [46, 49]. These studies demonstrate that one cannot compare expression differences identified using different microarray platforms without first cross-validating the methods for the specific genes of interest.
2.5. SUMMARY
The advent of technologies for expression profiling of multiple genes has launched a new era of biological research. Real time RT-PCR and DNA microarrays are among the most widely adopted methods employed in this new era. As originally developed, real-time RT-PCR and DNA microarrays were considered complementary technologies. Real-time RT-PCR is ideal for studies involving moderate numbers of genes (up to several hundred) in many biological specimens whereas DNA microarrays are better suited to analysis of many genes (tens of thousands) in fewer biological specimens. Given these characteristics DNA microarrays have more often been applied in the discovery phase of biological research with the aim of identifying the most informative genes. A relatively small number of genes, typically less than 50, can be identified whose differential expression is sufficient for the biological inquiry [50]. Once identified, the expression profile of this smaller set of informative genes can be screened with greater precision, better resolution, and more economically in a larger number of specimens by real-time RT-PCR. Concordance of results obtained from DNA microarray and real-time RT-PCR is critical if the former is used to identify smaller gene sets that will be screened subsequently by the latter technology. Existing data indicate that, although good overall correlations between technology platforms are possible, substantially different results can occur for individual genes. Thus, expression differences identified by microarrays must be verified if they are to be analyzed subsequently by another technology platform.
Microarray and real-time RT-PCR technology continues to evolve and improve. Increasingly, so-called “low density” microarrays targeting a small number of selected genes
GENE EXPRESSION PROFILING UTILIZING MICROARRAY TECHNOLOGY AND RT-PCR |
45 |
are being adopted in formats suitable for analyzing large numbers of specimens. Conversely, improvements in RT-PCR technology and instrumentation have enabled simultaneous analysis of larger gene sets. Thus, we expect that both of these technologies will be used in the future for gene “discovery” as well as for quantitative analysis of gene expression profiles.
ACKNOWLEDGEMENTS
John Morlan, Ken Hoyt, Debjani Dutta, Jennie Jeong, Anhthu Nguyen and Mylan Pho are thanked for providing microarray and TaqMan data. Chithra Sangli is thanked for assistance with statistical analysis and Joffre Baker is thanked for helpful discussions.
REFERENCES
[1]E.S. Lander et al. Nature, 409:860–921, 2001.
[2]J.C. Venter et al. Science, 291:1304–1351, 2001.
[3]L. Mancinelli, M. Cronin, W. Sadee. AAPS. Pharm Sci., 2:E4, 2000.
[4]A.D. Roses. Nature, 405:857–865, 2000.
[5]R. Higuchi, C. Fockler, G. Dollinger, and R. Watson. Biotechnology (N.Y.), 11:1026–1030, 1993.
[6]T.B. Morrison, J.J. Weis, and C.T. Wittwer. Biotechniques, 24:954–8, 960, 962, 1998.
[7]S. Nasarabadi, F. Milanovich, J. Richards, and P. Belgrader, Biotechniques, 27:1116–1118, 1999.
[8]J. Ju, C. Ruan, C.W. Fuller, A.N. Glazer, and R.A. Mathies. Proc. Natl. Acad. Sci. U.S.A, 92:4347–4351, 1995.
[9]S. Tyagi and F.R. Kramer. Nat. Biotechnol., 14:303–308, 1996.
[10]D. Whitcombe, J. Theaker, S.P. Guy, T. Brown, and S. Little. Nat. Biotechnol., 17:804–807, 1999.
[11]N. Thelwell, S. Millington, A. Solinas, J. Booth, and T. Brown. Nucleic Acids Res., 28:3752–3761, 2000.
[12]A. Solinas et al. Nucleic Acids Res., 29:E96, 2001.
[13]B.E. Caplin, R.P. Rasmussen, P.S. Bernard, and C.T. Wittwer. Biochemica, 1:5–8, 1999.
[14]E.A. Lukhtanov, I.V. Kutyavin, H.B. Gamper, R.B. Meyer, Jr. Bioconjug. Chem., 6:418–426, 1995.
[15]I. Afonina, I. Kutyavin, E. Lukhtanov, R. B. Meyer, and H. Gamper. Proc. Natl. Acad. Sci. U.S.A, 93:3199– 3204, 1996.
[16]I.V. Kutyavin et al. Nucleic Acids Res., 28:655–661, 2000.
[17]I.A. Afonina, M.W. Reed, E. Lusby, I.G. Shishkina, and Y.S. Belousov. Biotechniques, 32:940–949, 2002.
[18]E.A. Lukhtanov, M.A. Podyminogin, I.V. Kutyavin, R.B. Meyer, and H.B. Gamper. Nucleic Acids Res., 24:683–687, 1996.
[19]K.J. Livak and T.D. Schmittgen. Methods, 25:402–408, 2001.
[20]E. Southern, K. Mir, and M. Shchepinov. Nat. Genet., 21:5–9, 1999.
[21]B. Phimister. Nat. Genet., 21:1, 1999.
[22]M. Schena, D. Shalon, R.W. Davis, and P.O. Brown. Science, 270:467–470, 1995.
[23]M.B. Eisen and P.O. Brown. Methods Enzymol., 303:179–205, 1999.
[24]V.G. Cheung et al. Nat. Genet., 21:15–19, 1999.
[25]M.D. Kane et al. Nucleic Acids Res., 28:4552–4557, 2000.
[26]M. Beier and J.D. Hoheisel. Nucleic Acids Res., 28:E11, 2000.
[27]S. Singh-Gasson et al. Nat. Biotechnol., 17:974–978, 1999.
[28]T.R. Hughes et al. Nat. Biotechnol., 19:342–347, 2001.
[29]M. Baum et al. Nucleic Acids Res., 31:e151, 2003.
[30]S.P. Fodor et al. Science, 251:767–773, 1991.
[31]R.N. Van Gelder et al. Proc. Natl. Acad. Sci. U.S.A, 87:1663–1667, 1990.
[32]J. Eberwine et al. Proc. Natl. Acad. Sci. U.S.A, 89:3010–3014, 1992.
[33]U. Gubler and B.J. Hoffman, Gene, 25:263–269, 1983.
[34]S. Dudoit, R.C. Gentleman, and J. Quackenbush. Biotechniques, Suppl: 45–51, 2003.
46 |
DOMINICK SINICROPI, MAUREEN CRONIN AND MEI-LAN LIU |
[35]R.A. Irizarry et al. Nucleic Acids Res., 31:e15, 2003.
[36]M.A. Zapala et al. Genome Biol., 3:SOFTWARE0001, 2002.
[37]A. Richter et al. Biotechniques, 33:620–8, 630, 2002.
[38]J. Quackenbush, Nat. Genet., 32 Suppl:496–501, 2002.
[39]M.R. Weil, T. Macatee, and H.R. Garner. Biotechniques, 32:1310–1314, 2002.
[40]G. Stolovitzky. Curr. Opin. Struct. Biol., 13:370–376, 2003.
[41]T.R. Golub et al. Science, 286:531–537, 1999.
[42]A.A. Alizadeh et al. Nature, 403:503–511, 2000.
[43]P.J. van der Spek, A. Kremer, L. Murry, and M.G. Walker, Geno. Prot. and Bioinfo., 1:9–14, 2003.
[44]V.R. Iyer et al. Science, 283:83–87, 1999.
[45]M.S. Rajeevan, S.D. Vernon, N. Taysavang, and E.R. Unger, J. Mol. Diagn., 3:26–31, 2001.
[46]T. Yuen, E. Wurmbach, R.L. Pfeffer, B.J. Ebersole, and S.C. Sealfon, Nucleic Acids Res., 30:e48, 2002.
[47]B.J. van den et al. Am. J. Pathol., 163:1033–1043, 2003.
[48]J.K. Lee et al. Genome Biol., 4: R82, 2003.
[49]W.P. Kuo, T.K. Jenssen, A.J. Butte, L. Ohno-Machado, and I.S. Kohane, Bioinformatics., 18:405–412, 2002.
[50]C.H. Chung, P.S. Bernard, and C.M. Perou. Nat. Genet., 32 Suppl:533–540, 2002.
3
Microarray and Fluidic Chip for Extracellular Sensing
Mihrimah Ozkan1, Cengiz S. Ozkan2, Shalini Prasad1,
Mo Yang2, and Xuan Zhang2
1Department of Electrical Engineering, University of California Riverside, Riverside CA 92521
2Department of Mechanical Engineering, University of California Riverside, Riverside CA 92521
In the past two decades, the biological and medical fields have seen great advances in the development of biosensors and biochips capable of characterizing and quantifying biomolecules. Biosensors incorporate a biological sensing element that converts a change in an immediate environment to signals conducive for processing. Biosensors have been implemented for a number of applications ranging from environmental pollutant detection to defense monitoring. This chapter first provides an overview of the various types of biosensors and biochips that have been developed for biological applications, along with significant advances over the last several years in these technologies. It also describes the various classification schemes that can be used for categorizing the different biosensors and provides relevant examples of these classification schemes from recent literature. Finally it elucidates a sensing scheme based on cell based sensors. This technique is based on the development of single cell arrays that are used as biosensors that show parts per billion sensitivity and have the capability of identifying specific chemical analytes based on unique electrical identification tags also known as “Signature Patterns”. The reliability of this technique is verified using conventional fluorescence based techniques.
3.1. INTRODUCTION
According to a recently proposed IUPAC definition [136], “A biosensor is a selfcontained integrated device which is capable of providing specific quantitative or
48 |
MIHRIMAH OZKAN ET AL. |
semi-quantitative analytical information using a biological recognition element (biochemical receptor) which is in direct spatial contact with a transducer element. A biosensor should be clearly distinguished from a bioanalytical system, which requires additional processing steps, such as reagent addition. Furthermore, a biosensor should be distinguished from a bioprobe which is either disposable after one measurement, i.e., single use, or unable to continuously monitor the analyte concentration”. Biosensors that include transducers based on integrated circuit microchips are known as biochips [139].
Specificity and sensitivity are the main properties of any proposed biosensor. The first depends entirely on the inherent binding capabilities of the bioreceptor molecule whereas sensitivity will depend on both the nature of the biological element and the type of transducer used to detect this reaction [12]. In general, depending on the recognition properties of most biological components, two biosensor categories are recognized [48, 83, 119].
The first class of biosensors is the catalytic biosensors. These are also known as metabolism sensors and are kinetic devices based on the achievement of a steady-state concentration of a transducer-detectable species. The progress of the biocatalyzed reaction is related to the concentration of the analyte, which can be measured by monitoring the rate of formation of a product, the disappearance of a reactant, or the inhibition of the reaction. The biocatalyst can be an isolated enzyme, a microorganism, a sub cellular organelle, or a tissue slice. The second class of biosensors is the affinity biosensors. In these the receptor molecule binds the analyte “irreversibly” and non-catalytically. The binding event between the target molecule and the bioreceptor, for instance an antibody, a nucleic acid, or a hormone receptor, is the origin of a physicochemical change that will be measured by the transducer. Biosensor development is driven by the continuous need for simple, rapid, and continuous in-situ monitoring techniques in a broad range of areas. Biosensors can be classified according to either the nature of the bioreceptor element or the principle of operation of the transducer. The main types of transducer used in the development of biosensors can be divided into four groups [58] (1) optical, (2) electrochemical,
(3) mass-sensitive, and (4) thermometric. Each group can be further subdivided into different categories, because of the broad spectrum of methods used to monitor analyte–receptor interactions.
The bioreceptor component can be classified into five groups [114].
(1)Enzymes, proteins that catalyze specific chemical reactions. These can be used in a purified form or be present in a microorganism or in a slice of intact tissue. The mechanisms of operation of these bioreceptors can involve: (a) conversion of the analyte into a sensor-detectable product, (b) detection of an analyte that acts as enzyme inhibitor or activator, or (c) evaluation of the modification of enzyme properties upon interaction with the analyte.
(2)Antibodies and antigens. An antigen is a molecule that triggers the immune response of an organism to produce an antibody, a glycoprotein produced by lymphocyte B cells which will specifically recognize the antigen that stimulated its production (Aga 1997).
(3)Nucleic acids. The recognition process is based on the complementary nature of the base pairs (adenine and thymine or cytosine and guanine) of adjacent strands in the double helix of DNA. These sensors are usually known as genosensors.
MICROARRAY AND FLUIDIC CHIP FOR EXTRACELLULAR SENSING |
49 |
Alternatively, interaction of small pollutants with DNA can generate the recognition signal [140].
(4)Cellular structures or whole cells. The whole microorganism or a specific cellular component, for example a non-catalytic receptor protein, is used as the biorecognition element.
(5)Biomimetic receptors. Recognition is achieved by use of receptors, for instance, genetically engineered molecules [5, 10], artificial membranes [17], or molecularly imprinted polymers (MIP), that mimic a bioreceptor. The most recent investigations in artificial receptors include application of a combined approach of computer (molecular) modeling and MIP and the application of combinatorial synthesis for the development of new sensing layers.
Conventional methods for detecting environmental threats are primarily based on chemical, antibodyor nucleic acid-based assays. Biosensors incorporate a biological sensing element that converts a change in an immediate environment to signals conducive for processing. They generally rely on chemical properties or molecular recognition to identify a particular agent [98]. Modern approaches to biosensors can provide detection to a wide variety of analytes over a broad range of concentrations. However, as yet the current techniques involved in assessing risks to humans in areas contaminated with pollutants, pathogens or other agents are lacking technology. This is evidenced by our inability to: (1) simultaneously detect large numbers of possible threats, especially unknown or unanticipated ones;
(2) characterize the functionality of known agents or analytes that have been identified using conventional techniques; and (3) predict human performance decrements caused by low levels of agents or synergistic effects of environmental toxicants.
In the past ten years, substantial progress has been made in the functional characterization of drugs, pathogens and toxicants using cultured biological cells. This has resulted due to a growing interest in the use of biosensors in environmental [115], medical [145] toxicological [2, 7] and defense applications [98]. For researchers involved in the development of next generation sensors, biosensors have two intriguing characteristics. First, biosensor recognition elements need to make use of sensing elements that have a naturally evolved selectivity to biological or biologically active analytes. Second, biosensors need to have the capacity to respond to analytes in a physiologically relevant manner. Biosensors have the potential of providing rapid, sensitive, low-cost measurement technology for monitoring bioavailable analyte concentrations. Major issues impeding the widespread acceptance of biosensor technology have been previously identified as stability and reproducibility [84]. A desirable characteristic for any biosensor implementation is the capacity for continuous monitoring or, at a minimum, multiple use ability with little sensor regeneration or renewal. Associated, but not exclusive, features of ideal biosensors include rapid response times, automation, and portability [98].
In all the classes of biosensors discussed so far with the exception of cellular and tissue based biosensors there are many shortcomings the major one being that they are highly specific to the recognition of a specific analyte. A certain degree of success in terms of high selectivity and rapid response times have been achieved in the other biosensor categories chiefly due to a series of revolutionary advances in cell and molecular biology and technologies that have allowed for in-depth molecular interrogation. However, there is still
50 |
MIHRIMAH OZKAN ET AL. |
a major gap between our ability to perform functional assays using cells in the laboratory compared with in the field. This occurs due to the long response times associated with the molecular recognition elements, non-reusability of the detector performing functional assays and high specificity and excessive emphasis on ambient conditions for the accurate functioning of the sensors.
Cellular and tissue-based biosensors on the other hand incorporate isolated cells or tissue derived from a wide range of plant and animal sources. Also these biosensors use the cell as both the sensing as well as the transduction element. Thus elaborate signal transduction and coupling schemes can be eliminated and such sensors can be used in a diverse range of applications. Another added advantage of this category of sensors is that it is possible to monitor in-situ the physiological reaction to a particular chemical analayte along with simultaneous sensing. We elaborate on the sensing schemes using the other previously mentioned techniques of bioreceptor detection. We postulate the inherent drawbacks in each scheme and finally we elaborate upon the sensing technique based on detection and analysis of extra cellular potentials also known as “electrical sensing”.
3.2. ANTIBODY BASED BIOSENSORS
Antibody-based technology takes advantage of specific interactions with antigenic regions of an analyte to achieve high selectivity. Antibody-based approaches that require additional reagents for each measurement fall into the category of traditional immunoassays such as enzyme linked immunosorbant assay (ELISA), colorimetric test strips, etc. There are several approaches for detecting antigen: antibody binding ranging from conventional optical and piezoelectric [129] to more complex methods involving antibody-modified ion channel switches [21]. Fiber optic immunosensors detect binding of an analyte via modulation of evanescent wave properties yielding rapid and specific detection [131, 132]. Fiber optic sensors are particularly suited for applications where a low number of positive reactions are expected (drug screening or environmental sampling) since they can be reused multiple times as long as antibody sites remain unoccupied. Once significant positive signals are measured, the consumable elements of the immunosensor must be replaced. Challenges limiting the application of immunosensors include: antibody manufacturability, inherent antibody instability, and limited reversibility of binding. Technologies exploiting genetically engineered antibodies, e.g., phage display, [56] have demonstrated the most promise in addressing limitations in traditional antibody production. In addition, specific protein-binding nucleic acid sequences (aptamers) have been developed which may be well suited for sensor applications that previously relied on antibodies [109]. Whereas antibodies are raised in the circulation under in vivo conditions, aptamers can be evolved in the actual test media of interest. With regard to reusability, successful regeneration of immunosensors by chemical elution has been demonstrated as feasible, but not particularly practical in high throughput applications. 109. Commercially available systems such as a resonant mirror-based biosensor (Lab Systems) and a surface plasmon resonsance biosensor (BIAcore) utilize an automated fluidic regeneration system to recycle binding surfaces. There have been reports of exploiting the natural reversibility of antibodies for “recycling” sensors; (Hanbury et al., 1997) however, most immunosensors cannot operate continuously.
MICROARRAY AND FLUIDIC CHIP FOR EXTRACELLULAR SENSING |
51 |
3.3. NUCLEIC ACID BASED BIOSENSORS
Nucleic acid technology is based on the hybridization of known molecular DNA probes or sequences with complementary strands ina sample under test. Developments in sensors that exploit nucleic acid binding events (DNA sensors) have been generally limited to the antibody-based technology. Nucleic acid analysis in general requires extensive sample preparation, amplification, hybridization, and detection. In theory, nucleic acid analysis provides a higher degree of certainty than traditional antibody technologies because antibodies occasionally exhibit cross reactivity with antigens other than the analyte of interest. Near real time detection of hybridization events has been demonstrated in numerous optical [Pollard-Knight et al., 1997, 142] or electrochemical systems [24, 49, 85]. Further, the feasibility of sensor regeneration and reuse has been demonstrated in several opticalbased systems, including both fiber optic [41, Piunno et al., 1992] and resonant mirror applications [142]. In practice, however, development of nucleic acid sensor systems has been hampered by the challenges presented in sample preparation. Nucleic acid isolation remains the limiting step for all of the state-of-the-art molecular analyses. Typically, cells must be mechanically or chemically disrupted and treated with enzymes to remove associated proteins before nucleic acid can be isolated for hybridization to specific probes. As a result, rapid and automatable isolation of nucleic acid is an area of intense development at present.
3.4. ION CHANNEL BIOSENSORS
Membrane ion channels are targets of a range of transmitters, toxins, and potential pharmaceutical agentsThe fact that many ion channels and receptors can be purified and reconstituted in black lipid membranes (BLMs) for studies of function and pharmacology [86] has spurred initial interest in the development of channel/receptor-based biosensors. [74, 96, 126] However, ion channels especially those pertaining to mammalian physiology, cannot be considered robust in BLMs or isolated membrane patches due to the well-known property of ion channel “rundown” or “washout” [116]. In the absence of integral intracellular machinery provided by cells needed to maintain function, ion channels typical of mammalian physiology presently do not constitute practical biosensors.
3.5. ENZYME BASED BIOSENSORS
Enzyme-based technology relies upon a natural specificity of given enzymatic protein to react biochemically with a target substrate or substrates. Like ion channels, there are many enzymes that participate in cellular signaling and, in some cases, are targeted by compounds associated with environmental toxicity. In the medical diagnostic field, several manufacturers have marketed biosensors for measurement of common blood chemistry components including glucose, urea, lactate, and creatinine. ([64], Foch-Anderson et al., 1997). In general, enzyme-based biosensors employ semipermeable membranes through which target analytes diffuse toward a solid-phase immobilized enzyme compartment. The major drawback with this type of sensors is that many enzymes are inherently
