
Kluwer - Handbook of Biomedical Image Analysis Vol
.3.pdf
36 |
Farag et al. |
Table 1.2: Comparison between approximate times for single and composite
volume registration
|
|
|
Composite |
|
|
|
|
|
|
|
|
|
|
|
|
|
Single-Stage |
|
|
Surface Matching |
|
|
|
|||
|
No. of |
Volume |
Total time |
MI-GA |
||||
|
|
|
|
|||||
|
|
|
|
|||||
Case |
slices |
Features Extraction |
Matching |
Matching |
(sec) |
(sec) |
||
|
|
|
|
|
|
|
|
|
CT/CT |
236 |
20 sec |
30 sec |
100 sec |
150 |
|
300 |
|
CT/MR |
33/19 |
8 sec |
20 sec |
80 sec |
108 |
|
250 |
|
|
|
|
|
|
|
|
|
|
in a variety of clinically observable deficiencies, such as speech difficulties, pain, impairment of senses, loss of muscle control, and cognitive abnormalities [36]. In the brain, MS results in the inflammation and destruction of myelin, a fatty covering insulating nerve cells [36]. This damage results in decreased ability of the nervous system to control the body, leading to the clinically-observable symptoms of the disease. The causes of MS are not clearly accepted in the medical community. Geographic, genetic, and environmental factors all seem to be present [35]. Many researchers have proposed that MS is an autoimmune disease [35]. Though there are promising research developments, at this time, no cure is known for this disease. Several treatment options are available, and allow for management of the disease. Despite this, most patients progress in disability over the course of their life [35]. Though not usually a fatal disease in and of itself, the resulting disabilities may contribute to accidental mishaps [35].
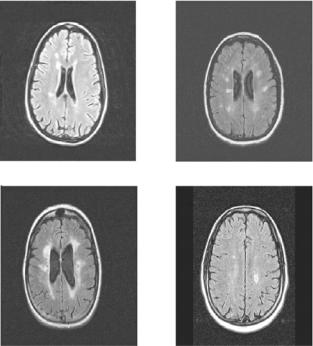
When patients with MS are imaged using MRI modalities, lesions (also referred to as plaques or deficits) can be contrasted against surrounding, normal brain tissue, by choice of appropriate scan parameters, and depending on the state of the lesion [37]. Figure 1.14 shows several slices of different FLAIR MR images of patients with MS. MS lesions appear as hyper intense regions in the patient’s brain.
The use of MR imagery in the evaluation of MS involves the identification of abnormal brain tissue (MS lesions), and normal, non-diseased brain tissue (gray and white matter). MRI has been found to be a sensitive marker to changes in disease progression, and evaluation of MRI studies of MS patients can be useful as an outcome measurement in MS studies [4]. Despite these statements, evaluation of the disease cannot be completely based upon MRI findings. MS lesion activity observed in MRI studies of the brain does not always correspond
Medical Image Registration |
37 |
Figure 1.14: Sample slices from FLAIR MRI studies of patients with MS. Hyperintense regions in the brain are indicative of plaques caused by MS.
to clinically observed deficits [37]. As well, quantification of MRI studies of MS have yet to be perfected, and most studies of MS do not generate enough MRI data to evaluate the nuances in the application of MRI to study MS.
It is desirable to develop computer tools to assist experts in the study of MS using MR imaging. Allowing a computer to automatically identify normal and abnormal brain tissue would free an expert from the arduous task of manually examining each slice of a study, while generally increasing the reproducibility of the identification by removing the subjectivity of the human observer. Medical registration tools would also be useful for automatic, retrospective alignment of patient studies, taken at different points in time, to allow for qualitative comparison of different studies of a patient of the course of his or her treatment. This type of analysis would aid the expert in deciding if the disease is responding well to the present treatment, or if a change in the treatment is warranted.
Typically in an MRI study, a patient is placed in the scanner with little regard for positioning of the anatomy of interest. The only constraints are that the
38 |
Farag et al. |
anatomy of interest falls within the scanning volume, and that the patient is generally placed in some orientation such that gross anatomical features are placed in some direction (for example, the patient’s nose points upward in the scanner). In the context of qualitative evaluation of MS studies however, this arrangement leads to hindrances in evaluation, due to the fact that multiple scans must be compared with one another. Due to the largely arbitrary positioning of the anatomy in the scanner, in a slice-by-slice comparison between studies, quite different anatomy can by chance be located on the same slice numbers in different studies.
It is desirable to be able to perform registration using the intrinsic approaches, rather than imposing limitations in the scanning procedure, or affixing artificial fiducial markers on the patient’s head. For best accuracy, artificial markers would likely be affixed to the skull, and therefore would be inconvenient and potentially painful for the patient. Additionally, this procedure would also introduce a risk of infection. Furthermore, using intrinsic registration techniques, it is also desirable to be able to apply registration retroactively, allowing for current data sets to be aligned with data sets taken previously in a patient’s history, or perhaps with an imaging modality that prevents the use of artificial markers. In the study of MS using MRI, for comparison of scans taken at different points of time in a clinical study, of the same patient, a registration technique is necessary. Such a tool would allow for alignment of the patient’s anatomy in different scans. When this alignment is accomplished, qualitative comparison of scans becomes easier to an expert viewer, as image slices will now contain the same anatomy, and quantitative comparison between studies is enabled in the same manner. Registration can also be used to assist in segmentation. For example, if a model of the patient’s anatomy is known, then a study can be registered to that model, allowing for segmentation of certain classes of problems to be made trivial, as the segmentation of the data set is then known a priori from the model. In the MS research, this approach is used for segmentation of a patient’s brain from his head. In this context, registration is performed by maximization of mutual information. This technique has generally been found to perform well, and is useful in the MS clinical settings. This technique was studied, implemented, and tested using MS patient studies. Additionally, the performance of this method was enhanced by application of parallel programming techniques.
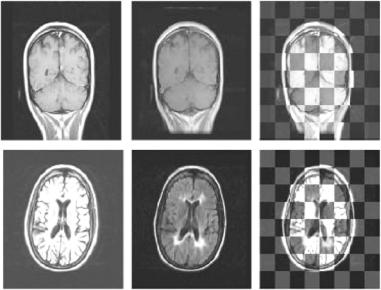
Figure 1.15 shows sample slices from the registration studies. Each row of the figure corresponds to a single study. The first column of the figure shows a sample slice from the floating volume used for each study. The second column of
Medical Image Registration |
39 |
Figure 1.15: Sample registration results from each samples of MS data sets. The first column is a sample slice from the floating volume used. The second column is the corresponding slice from the re-sampled reference volume. The third column is the checkerboard composite image of the two corresponding slices from the floating and resampled reference volumes. The floating volume and reference volumes used in each trial were from the same patient.
the figure shows the corresponding slice from the re-sampled reference volume. Finally, the third column of the figure shows the checkerboard composite image formed by fusion of the corresponding floating and re-sampled reference volume slices.
1.6.3 Lung Cancer Application
Another practical application for monomodal registration in the lung cancer diagnosis. Lung cancer remains the leading cause of mortality cancer. In 1999, there were approximately 170,000 new cases of lung cancer [38]. The five-year survival rate from the diseases is 14% and has increased only slightly since the early 1970s despite extensive and costly research efforts to find effective therapy. The disparity in survival between early and late-stage lung cancer is substantial, with a five-year survival rate of approximately 70% in stage 1A disease compared to less than 5% in stage IV disease according to the recently revised lung cancer
40 |
Farag et al. |
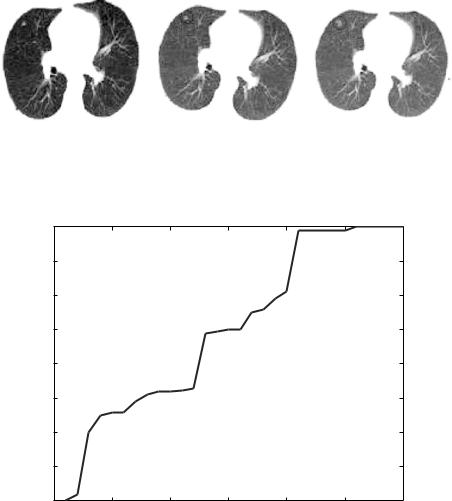
Figure 1.16: (a) CT scan June 2000 (b) CT scan June 2001 (c) CT scan June
2002. |
|
|
|
|
|
|
I(α)0.9 |
|
|
|
|
|
|
0.8 |
|
|
|
|
|
|
0.7 |
|
|
|
|
|
|
0.6 |
|
|
|
|
|
|
0.5 |
|
|
|
|
|
|
0.4 |
|
|
|
|
|
|
0.3 |
|
|
|
|
|
|
0.2 |
|
|
|
|
|
|
0.1 |
5 |
10 |
15 |
20 |
25 |
30 |
0 |
Generation number
Figure 1.17: MI fitness function at different GA iterations.
staging criteria [38]. The disproportionately high prevalence and mortality of lung cancer has encouraged attempts to detect early lung cancer with screening programs aimed at smokers. Smokers have an incidence rate of lung cancer that is 10 times that of nonsmokers and accounts for more than 80% of lung cancer cases in the United States [38]. One in every 18 woman and every 12 men develop lung cancer, making it the leading cause of cancer deaths. Early detection of lung tumors (visible on chest film as nodules) may increase the patient’s chance of survival.

Medical Image Registration |
41 |
Figure 1.18: (a) Result of registration of data in Figs. 1.16.a and 1.16.b. (b) Result of registration of data in Figs. 1.16.a and 1.16.c.
The Jewish Hospital Foundation in Louisville, KY., proposed a cancer screening and early detection study in a randomized trial with the following specific aims: (1) to determine whether the use of spiral CT scanning of the chest detects early lung abnormalities that lead to cancer, which are not visible on chest X-rays in patients at high-risk for developing lung cancer; and (2) to determine whether annual spiral chest CT scans of the chest in high-risk patients result in an improvement in survival. To test and prove these hypothesis, data was collected from 1000 symptomatic patients above 60 years of age with positive smoking history will undergo screening with low dose spiral CT (LDCT) and chest radiography. Screening was performed every three months on the selected 1000 symptomatic patients. The role of the image registration process was to help in studying the development of abnormalities.
Again, maximization of mutual information enhanced by using genetic algorithms was used in this application. Figure 1.16 shows different CT images for the same patient taken at various periods. Registration of these images using the mutual information criterion was performed fully automatically on a PC computer with microprocessor 2.4 GHz. The algorithm took less than 8 min. to register all data. The recovered rotational transformation parameters were generally smaller than 15 degrees, while the translational parameters varied up to 20 mm. Figure 1.17 shows how the GA iterations improve the MI measure overtime until maximum fitness is reached. Figure 1.18 shows the results of
42 |
Farag et al. |
registration for the data shown in Fig. 1.16. The registration process was able to show that new parts of abnormality appeared in later scans between the period June 2001 to June 2002.
Questions
1.Based on the classification defined in this chapter, define all the possible classifications of the following registration techniques:
(a)Registration by maximization of Mutual Information.
(b)Surface Signature registration.
(c)Grid Closest Point registration.
2.Using the formulation for the Surface Signature images, derive and draw the signature image for the following parametric shapes. (choose points of interest on each shape)
(a)a sphere of radius r
|
r cos(2π u) |
|
Sph(u, v) |
r sin(2π u)cos(2π v) |
|
|
|
|
|
= r sin(2π u)sin(2π v) |
|
(b)a cylinder of radius r and height h
r cos(2π u)
C yl(u, v) |
|
sin(2 ) |
|
|
= r |
v/ hπ u |
|
(1.21)
(1.22)
3.Describe how the Genetic Algorithm technique can be used as an optimizer for the MI registration technique.
4.Derive Eq. (1.19) from Eq. (1.20).

Medical Image Registration |
43 |
Bibliography
[1]Maintz, J. B. A. and Viergever, M. A., A survey of medical image registration, Medical Imaging Analysis, Vol. 2, no. 1, pp. 1–36, 1998.
[2]van den Elsen, P. A. and Viergever, M. A., Automated CT and MR brain image registration using geometrical feature correlation., Proc. IEEE
Nuclear Science Symposium and Medical Imaging Conference,
pp.1827–1830, 1993.
[3]Yamany, S. M., Ahmed, M. N., and Farag, A. A., Novel surface registration using the grid closest point (GCP) transform, Proc. IEEE International Conference on Image Processing, Chicago, October 1998.
[4]Besl, B. J. and McKay, N. D., A method for registration of 3D shapes, IEEE Transactions on Pattern Analysis and Machine Intelligence, Vol. 14, no. 2, pp. 239–256, 1992.
[5]Eggert, D. W., Fitzgibbon, A. W., and Fisher, R. B., Simultaneous registration of multiple range views for use in reverse engineering of CAD models, Computer Vision and Image Understanding, Vol. 69, no. 3, pp. 253– 272, March 1998.
[6]Zhang, Z., Iterative point matching for registration of free-form curves and surfaces, International Journal of Computer Vision, Vol. 13, no. 2,
pp.119–152, 1994.
[7]Chen, Y. and Medioni, G., Object modelling by registration of mutliple range images, Image Vision Computing, Vol. 10, no. 3, pp. 145–155, 1992.
[8]Bergevin, R., Laurendeau, D., and Poussart, D., Registering range views of multipart objects, Computer Vision and Image Understanding, Vol. 61, no. 1, pp. 1–16, January 1995.
[9]Yamany, S. M., Ahmed, M. N., and Farag, A. A., A New genetic-based technique for matching 3D curves and surfaces, Pattern Recognition, Vol. 32, no. 10, p. 1817–1820, 1999.
[10]Champleboux, G., Lavallee, S., Szeliski, R., and Burnie, L., From accurate range imaging sensor calibration to accurate model-based 3D
44 |
Farag et al. |
object recognition, Proc. IEEE Computer Vision and Pattern Recogni-
tion (CVPR), Champaign, pp. 83–89, 1992.
[11]Brujic, D. and Ristic, M., Analysis of free form surface registration, Proc IEEE International Conference on Image Processing (ICIP), Lausanne, Vol. 17, p. 2–5, 1996.
[12]Menq, C. H., Yau, H. T., and Lai, G. Y., Automated precision measurement of surface profile in CAD-directed inspection, IEEE Transactions on Robotics Automation, Vol. 8, pp. 268–278, 1992.
[13]Blais, G. and Levine, M. D., Registering multiview range data to create 3D computer objects, IEEE Transactions on Pattern Analysis and Machine Intellegence, Vol. 17, pp. 820–824, 1995.
[14]Masuda, T. and Yokoya, N., A robust method for registration and segmentation of multiple range images, Computer Vision and Image Understanding, Vol. 61, pp. 295–307, 1995.
[15]Lu, F. and Milios, E. E., Robot pose estimation in unknown environments by matching 2D range scans, Proc. IEEE Computer Vision and Pattern Recognition (CVPR), Seattle, pp. 935–938, 1994.
[16]Tarel, J.-P. and Boujemaa, N., A coarse to fine 3D registration method based on robust fuzzy clustering, Computer Vision and Image Understanding, Vol. 73, no. 1, pp. 14–28, 1999.
[17]Neri, F. and Saitta, L., Exploring the power of genetic search in learning symbolic classifiers, IEEE Transactions on Pattern Analysis and Machine Intellegence, Vol. 18, no. 11, pp. 1135–1141, 1991.
[18]Grefenstette, J. J., Optimization of control parameters for genetic algorithms classifiers, IEEE Transactions on Systems, Man, and Cybernatics, Vol. 16, no. 1, pp. 122–128, January 1996.
[19]Fourman, M. P., Compaction of symbolic layout using genetic algorithms, Proc. of International Conference of Genetic Algorithms and their Applications, pp. 141–153, 1985.
[20]DeJong, K., Learning with genetic algorithms: An overview, Machine Learning, Vol. 3, pp. 121–138, 1988.
Medical Image Registration |
45 |
[21]Ankenbrandt, C. A., Buckles, B. P., and Petry, F. E., Scene recognition using genetic algorithms with semantic nets, Pattern Recognition Letters, Vol. 11, pp. 285–293, 1990.
[22]Bhanu, B. and Lee, S., Genetic Learning for Adaptive Image Segmentation, Kluwer Academic Publishers, Dordrecht, 1994.
[23]Holland, J. H., Adaptation in Natural and Artificial Systems, University of Michigan Press, Michigan, 1975.
[24]Goldberg, D. E., Genetic Algorithms in Search, Optimization and Machine Learning, Addison-Welsey, Reading, MA, 1989.
[25]Wells, W. M., Viola, P., Atsumi, H., Nakajima, S., and Kikinis, R., Multimodal volume registration by maximization of mutual information, Medical Imaging Analysis, Vol. 1, no. 1, pp. 35–54, March 1996.
[26]Yamany, S. M. and Farag, A. A., Surface signatures: An orientation independent free-form surface representation scheme for the purpose of objects registration and matching, IEEE Transactions on Pattern Analysis and Machine Intellegence, Vol. 24, no. 8, pp. 1105–1120, 2002.
[27]Stein, F. and Medioni, G., Structural indexing: Efficient 3D object recognition, IEEE Transactions on Pattern Analysis and Machine Intellegence, Vol. 14, no. 2, pp. 125–145, 1992.
[28]Chua, C. S. and Jarvis, R., Point signatures: A new representation for 3D object recognition, International Journal of Computer Vision, Vol. 25, no. 1, pp. 63–85, 1997.
[29]Dorai, C. and Jain, A. K., COSMOS; A representation scheme for 3D free-form objects, IEEE Transactions on Pattern Analysis and Machine Intellegence, Vol. 19, no. 10, pp. 1115–1130, October 1997.
[30]Johnson, A. and Hebert, M., Surface matching for object recognition in complex three-dimensional scenes, Image and Vision Computing, Vol. 16, pp. 635–651, 1998.
[31]Maes, F., Collignon, A., Vandermeulen, D., Marchal, G., and Suetens, P., Multimodality image registration by maximization of mutual
