
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index

St John’s Wort
Summary and Pharmaceutical Comment
The chemical composition of St John’s wort has been well studied. Documented pharmacological activities provide supporting evidence for several of the traditional uses stated for St John’s wort. Many pharmacological activities appear to be attributable to hypericin, hyperforin and/or the flavonoid constituents; hypericin is also reported to be responsible for the photosensitive reactions that have been documented for St John’s wort. With regard to the antidepressant effects of St John’s wort, hyperforin rather than hypericin, as originally thought, has emerged as one of the major constituents responsible for antidepressant activity. However, further research is required in order to determine which other constituents contribute to the antidepressant effect.
There are now over forty clinical trials of H. perforatum preparations involving patients with different types of depression, although a rigorous systematic review and metaanalysis found that overall the evidence is inconsistent and complex. H. perforatum preparations and standard antidepressant agents appear to show similar effects, whereas H. perforatum preparations have only small benefits over placebo in patients with major depression; in older studies in patients with mild-to-moderate depression, H. perforatum preparations appear to be of more benefit than placebo. A previous systematic review/meta-analysis, for which inclusion criteria for trials were slightly less strict, found evidence from randomised, controlled trials that St John’s wort preparations were more efficacious than placebo in the treatment of mild- to-moderately severe depression.
An important point is that there is heterogeneity not only among the trials and their results, but also among the different manufacturers’ products tested. Products are not necessarily equally effective, and the results of the analyses above should not be extrapolated to other H. perforatum preparations, which may differ considerably in their pharmaceutical quality. These comments also imply that the need for dose adjustment should be considered for patients changing from one St John’s wort product to another.
Clinical safety data from randomised controlled trials, systematic reviews and meta-analyses of trials, and postmarketing surveillance and other observational studies, indicate that certain St John’s wort extracts are well-tolerated when taken at recommended doses for shorter periods of time (around eight weeks). Certain St John’s wort preparations do appear to have a more favourable safety profile, at least with short-term use, than standard antidepressants, particularly older antidepressant agents, a factor that may be important in patients continuing to take medication. Data from the small number of longer-term (one year) studies support the tolerability of certain St John’s wort extracts, although further investigation of long-term use is warranted. Adverse events/ effects reported are generally mild and most commonly gastrointestinal symptoms. These observations, however, are based on data collected in the settings of formal randomised or observational studies, and usually where H. perforatum
has been prescribed under the supervision of a physician. The safety of St John’s wort products taken as self-treatment without supervision by a healthcare professional requires further study.
The risk of photosensitive reactions following oral ingestion of St John’s wort preparations appears to be low, since serum and skin concentrations of hypericin (the photosensitising constituent) after oral administration of recommended doses are below 100 ng/mL, although caution is advised as it is possible that there may be unusual absorption of hypericin in some individuals and in fair-skinned individuals and after extended periods of solar irradiation, there may be increased susceptibility to the photosensitising properties of hypericin. Likewise, the phototoxic potential of topical application of H. perforatum preparations appears to be low, although caution is necessary, particularly as hypericin may penetrate more highly through broken or lesional skin, and there may be increased susceptibility to the photosensitising properties of hypericin in fair-skinned individuals and after extended periods of solar irradiation.
There are important pharmacokinetic interactions between St John’s wort preparations and certain other medicines, leading to a loss of or reduction in the therapeutic effect of those medicines, and potential for important pharmacodynamic interactions, which could lead to enhancement or antagonism of pharmacological effects, depending on the activities of the co-administered medicines. Drugs that may be affected by pharmacokinetic interactions include certain anticonvulsants, ciclosporin, digoxin, indinavir (and other HIV protease inhibitors), oral contraceptives, theophylline and warfarin, and by pharmacodynamic interactions, triptans and selective serotonin reuptake inhibitors. Advice is that patients taking these medicines should stop taking St John’s wort preparations, generally after seeking professional advice, as dose adjustment may be necessary.
There is evidence that pharmacokinetic interactions arise through induction, by constituents of St John’s wort
preparations, of the cytochrome P450 (CYP) drug- S metabolising enzyme CYP3A4 (and possibly certain other CYP
enzymes), and through effects on P-glycoprotein (a transport protein). As CYP3A4 is involved in the metabolism of many drugs, and as P-glycoprotein is involved widely in drug transport, it is possible that St John’s wort preparations interact with other medicines in addition to those already identified.
In view of the lack of toxicity data, St John’s wort preparations should not be used during pregnancy and lactation.
Pharmacists and other healthcare professionals should be mindful that patients at all levels of health care may self-treat with herbal and other non-prescription medicines, and that use is not necessarily disclosed to healthcare professionals. St John’s wort products may be used in addition to, or instead of, standard antidepressants and other conventional medicines. The prevalence of concurrent use of St John’s wort products
549
550 St John’s Wort
and antidepressant medicines may be particularly high among certain subgroups of patients with depression: a crosssectional survey involving members of a depression self-help group reported that over 50% were using St John’s wort and that, among these individuals, the concurrent use of St John’s wort and conventional antidepressants was 29% (see Sideeffects, Toxicity, Clinical data).
Species (Family)
Hypericum perforatum L. (Guttiferae/Clusiaceae)
Synonym(s)
Hypericum, Hypericum veronense Schrank, H. noeanum Boiss., Millepertuis
Part(s) Used
Herb
Pharmacopoeial and Other Monographs
American Herbal Pharmacopoeia(G1)
BHMA 2003(G66)
BHP 1996(G9)
BP 2007(G84)
Complete German Commission E(G3)
ESCOP 2003 (G76)
Martindale 35th edition(G85)
Ph Eur 2007(G81)
USP29/NF24(G86)
WHO volume 2 (2002)(G70)
Legal Category (Licensed Products)
GSL (for external use only)(G37)
Constituents
See also References 1 and 2 and General References G1 and G2.
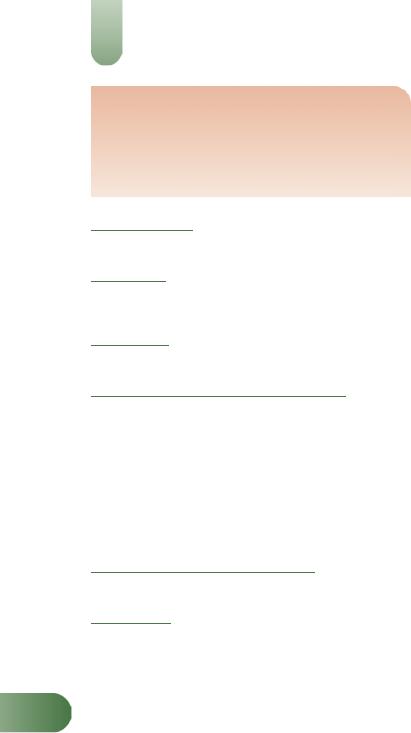
Anthraquinone derivatives (naphthodianthrones) Hypericin, pseudohypericin and isohypericin; protohypericin and protopseudohypericin (biosynthetic precursors of hypericin and pseudohypericin, respectively) are present in fresh material.
SCyclopseudohypericin is also stated to be present. The hypericin content (approximately 0.1–0.15%) includes both hypericin and pseudohypericin(3) and is sometimes referred to as 'total hypericins'.
Flavonoids Flavonols (e.g. kaempferol, quercetin), flavones (e.g. luteolin) and glycosides (e.g. hyperoside, isoquercitrin, quercitrin, rutin), biflavonoids including biapigenin (a flavone) and amento-
flavone (a biapigenin derivative)(4, 5) and catechins (flavonoids often associated with condensed tannins).(6, 7) The concentrations of rutin, hyperoside and isoquercitrin have been reported as 1.6, 0.9 and 0.3%, respectively.(8)
Prenylated phloroglucinols Hyperforin (2.0–4.5%) and adhyperforin (0.2–1.9%).(6, 9, 10, G1)
Tannins 8–9%. Type not specified. Proanthocyanidins (condensed type) have been reported.(G2)
Other phenols Caffeic, chlorogenic, p-coumaric, ferulic, p- hydroxybenzoic and vanillic acids.
Volatile oils 0.05–0.9%. Major component (not less than 30%) is methyl-2-octane (saturated hydrocarbon); others include n-non- ane and traces of methyl-2-decane and n-undecane (saturated hydrocarbons),(11) a- and b-pinene, a-terpineol, geraniol, and traces of myrcene and limonene (monoterpenes), caryophyllene and humulene (sesquiterpenes).(12, 13)
Other constituents Acids (isovalerianic, nicotinic, myristic, palmitic, stearic), carotenoids, choline, nicotinamide, pectin, b-
sitosterol, straight-chain saturated hydrocarbons (C16, C30)(11, 14) and alcohols (C24, C26, C28).(11, 14)
Quality of plant material and commercial products
According to the British and European Pharmacopoeias, St John's wort herb consists of the whole or cut dried flowering tops of H. perforatum, harvested during flowering time, and contains not less
than 0.08% of total hypericins, expressed as hypericin, calculated with reference to the dried drug.(G81, G84) Detailed descriptions of
H. perforatum herb for use in botanical, microscopic and macroscopic identification have been published, along with
qualitative and quantitative methods for the assessment of H. perforatum herb raw material.(G1)
As with other herbal medicinal products, there is variation in the qualitative and quantitative composition of commercial St John's wort preparations. In the USA, analysis of 21 St John's wort products (18 of which claimed to contain only standardised extracts and three of which were combinations of extracts and crude plant material) found that seven products did not meet at least one of the quality criteria assessed: four products had a hypericin content ranging from 77% to 85% of that stated on their labels or, if not stated on the label, of the minimum acceptable concentration permitted in the analysis; one of the two products claiming to contain hyperforin contained only 21.7% of the stated amount; five products contained cadmium exceeding acceptable concentrations in the analysis, and two of these
products contained more than twice the permitted concentration of cadmium.(15) Overall, five of the 18 products labelled with
hypericin and/or hyperforin concentrations, and five of the seven products labelled as being produced from 'aerial parts', failed the analysis. Another analytical study which investigated eight St John's wort products marketed in the USA found that their hyperforin content varied from 0.01–1.89%, and that only two products contained more than one percent hyperforin, the minimum concentration suggested to be required for antidepressant effects.(16) Similarly, hypericin content varied from 0.03– 0.29% and, for several products, the actual content ranged from 57–130% of that stated on the label.
Analysis (using a liquid chromatographic method with fluorescent detection) of 54 commercially available St John's wort products purchased in the USA and Canada found that only two of the products had a total naphthodianthrone concentration (hypericin and pseudohypericin) within 10% of that stated on the label.(17) Overall, total naphthodianthrone content for tablets and capsules, respectively, ranged from 0–108.6% and from 31.3– 80.2% of the amount stated on the label. The naphthodianthrone content of products formulated as tinctures ranged from 0– 118.6 mg/mL.
Isolated hyperforin is susceptible to oxidation, although the hyperforin content of dried herb and preparations containing extracts of H. perforatum appears to be more stable.(18) Degradation products for hyperforin include deoxyfurohyperforin A,(19) furohyperforin, furoadhyperforin and furohyperforin A and other oxygenated hyperforin analogues.(20, 21)
A fast high-performance liquid chromatographic (HPLC) method with photodiode array detection has been developed for the determination of six of the naphthodianthrone (including hypericin) and phloroglucinol (including hyperforin) compounds in H. perforatum extracts.(22) The method allows quantitative determination of concentrations as low as 2 mg/mL and 0.5 mg/mL for hyperforin and hypericin, respectively, while detection limits for these compounds were 0.1 and 0.02 mg/mL, respectively.
Variation in St John's wort products is not limited to product composition, but as with other herbal medicinal products also applies to the dissolution profiles of products which might be considered to be pharmaceutically equivalent. In vitro dissolution tests using biorelevant conditions (i.e. simulated gastric fluid and similar substances as the dissolution media) were used to determine the dissolution profiles of five St John's wort products purchased in Germany. All products were ethanol or methanol
extracts of St John's wort, formulated as tablets or capsules and contained 300–612 mg extract per unit dosage form.(23) In
simulated gastric fluid without pepsin, and under conditions simulating the fasted state in the proximal small intestine, dissolution of hyperforin was 'not detected' and 'poor', respectively. Dissolution of hyperforin improved under conditions simulating postprandial conditions in the proximal small intestine for some products (e.g. 90% release within two hours), but was relatively poor for other products (e.g. <50% release within two hours). Dissolution of hypericins into simulated gastric fluid was also not detected; results for dissolution of hypericins into other media were either not available or were deemed to be unreliable. Dissolution of the flavonoid compounds tested (rutin, hyperoside, isoquercitrin and quercitrin) was good into all media tested, although there were variations between products in the release of rutin.(23) These results indicate that these St John's wort products cannot be considered interchangeable; the implication being that the need for dose adjustment should be considered for patients changing from one St John's wort product to another.
Food Use
St John's wort is listed by the Council of Europe as a natural source of food flavouring (herb: category 5).(G17)
Herbal Use
St John's wort is stated to possess sedative and astringent properties. It has been used for excitability, neuralgia, fibrositis,
sciatica, wounds, menopausal neurosis, anxiety and depression and as a nerve tonic.(G3, G7, G32) St John's wort also has a long
history of traditional use in topical preparations for wound healing.(G1, G7) St John's wort is used extensively in homeopathic preparations as well as in herbal products. Modern interest is focused on its use as an antidepressant.
Dosage
Dosages for oral administration (adults) recommended in standard herbal reference texts(G6, G7) are the same for several traditional uses; examples are given below.
Dried herb 2–4 g as an infusion three times daily.(G7)
Liquid extract 2–4 mL (1 : 1 in 25% alcohol) three times daily.(G7)
Tincture 2–4 mL (1 : 10 in 45% alcohol) three times daily.(G7) Doses of St John's wort extracts used in clinical trials involving
patients with mild to moderate depression generally range from
St John’s Wort |
551 |
Figure 1 Selected constituents of St John’s wort.
240–1800 mg daily (equivalent to varying concentrations of
hypericin and hyperforin, depending on the extract), typically for four to six weeks.(24)
Pharmacological Actions
The major active constituents are considered to be hyperforin (a prenylated phloroglucinol) and hypericin (a naphthodianthrone),
although other biologically active constituents, e.g. flavonoids and S tannins, are also present.(25) Several pharmacological activities,
including antidepressant, anticancer, antiviral and antibacterial effects, have been documented for extracts of St John's wort and/ or its constituents following preclinical studies. Clinical studies mainly have described antidepressant effects for St John's wort preparations.
In vitro and animal studies
Pharmacokinetics In vivo (rats), the bioavailability of hypericin following oral administration was increased by co-administration of procyanidin B2, isolated from H. perforatum, or hyperoside.(26)
Antidepressant activity The precise mechanism of action for the antidepressant effect of St John's wort is unclear. Initially, attention was focused on hypericin as the constituent of St John's wort believed to be responsible for the herb's antidepressant effects. Inhibition of monoamine oxidase (MAO) type A and B in

552 St John’s Wort
Figure 2 St John’s wort (Hypericum perforatum).
rat brain mitochondria in vitro was described for hypericin.(27)
However, other studies have demonstrated only weak or no MAO inhibition.(28–30)
In vitro receptor binding and enzyme inhibition assays carried out using hypericum extract demonstrated significant receptor affinity for adenosine, GABAA, GABAB, benzodiazepine and MAO types A and B, although, with the exception of GABAA and GABAB, the concentrations of hypericum required were unlikely to be attained after oral administration in humans.(31) Other biochemical studies have reported that the hypericum extract LI 160 is only a weak inhibitor of MAO-A and MAO-B activity, but that it inhibits the synaptosomal uptake of serotonin (5- hydroxytryptamine or 5-HT), dopamine and noradrenaline (norepinephrine) with approximately equal affinity and also leads to a downregulation of b-receptors and an upregulation of 5-HT2 receptors in the rat frontal cortex.(32) The effects of fluoxetine and hypericinand flavonoid-standardised hypericum extracts (LI 160, 0.3% hypericin and 6% flavonoids and Ph-50, 0.3% hypericin and 50% flavonoids) on the concentrations of neurotransmitters in brain regions were studied in rats.(33) All three preparations induced a significant increase in 5-HT concentrations in the rat cortex, both LI 160 and Ph-50 caused increases in noradrenaline (norepinephrine) and dopamine in the rat diencephalon and Ph-50 also induced an increase in the noradrenaline (norepinephrine)
content in the brainstem, areas that are implicated in depression.(33)
SOther in vitro experiments using peripheral blood mononuclearcells have shown that an alcoholic extract of H. perforatum
containing 0.25 mg/mL hypericin downregulated mitogenmediated tryptophan degradation in a concentration-dependent manner.(34) Tryptophan is a precursor for biosynthesis of 5-HT.
Hyperforin has now emerged as being one of the major active constituents of importance in antidepressant activity.(35) Hyperforin has been shown to be an uptake inhibitor of 5-HT, dopamine, noradrenaline (norepinephrine), GABA and L-gluta- mate in synaptosomal preparations(36) and to inhibit 5-HT uptake in rat peritoneal cells in a dose-dependent manner.(37) Studies have also described discrepancies between observed and theoretical IC50 values, indicating that hyperforin is not the only component of hypericum extract that is responsible for the observed effects.(37, 38) Adhyperforin, another phloroglucinol constituent of H. perforatum, also inhibits the uptake of dopamine, serotonin and norepinephrine in vitro, and its possible involvement in the antidepressant activity of St John's wort requires further investigation.(39)
Figure 3 St John’s wort – dried drug substance (herb).
In vitro screening of the activities of hypericin, pseudohypericin, hyperforin and several flavonoid constituents of H. perforatum using 42 biogenic amine receptors and transporters (available as part of the National Institute of Mental Health Psychoactive Drug Screening Program of the USA) showed that compounds significantly inhibited ligand binding at the following receptors in particular: amentoflavone – serotonin (5-HT1D, 5- HT2C), dopamine-D3, opiate (delta), benzodiazepine; hypericin and pseudohypericin – dopamine-D3, dopamine-D4, b-adrenergic; hyperforin – dopamine-D1, dopamine-D5.(40) Hyperforin was less active than the other constituents tested on all receptors screened. Screening revealed some interactions at G-protein coupled receptors that were previously unreported (e.g. hypericin and b- adrenergic activity).
The effects of hyperforin on interleukin-6 (IL-6) release in different experimental models have been explored as a possible alternative mechanism for antidepressant effects, since St John's wort has been shown to inhibit substance-P mediated effects (substance P has been implicated in the aetiology of depression).(41) Hyperforin inhibited both substance-P- and lipopoly- saccharide-induced IL-6 release in human astrocytoma cells (IC50 = 1.6 and 1.9 mmol/L, respectively), although concentrations required to achieve this were around one order of magnitude higher than those found in the plasma of patients treated with H. perforatum extracts.(42)
Other findings indicate that flavonoids, as well as hyperforin and hypericin, are at least some of the constituents of H. perforatum responsible for its antidepressant activity.(43, 44)
In vivo (mice and rats) experiments which assessed the effects of a hydroalcoholic extract of H. perforatum (containing hypericin 0.15% and hyperforin 3.2%) and the same extract with hypericin and hyperforin removed in turn, showed that all extracts, including the extract devoid of both hypericin and hyperforin
(but containing 12% flavonoids), retained activity in behavioural models.(43)
It has been reported that the mode of action of hyperforin in serotonin uptake inhibition seems to be associated with the elevation of free intracellular sodium ion concentrations(45) and that this may be secondary to activation of the Naþ/Hþ exchange as a result of a decrease in intracellular pH.(46) Further in vitro experiments using two cell systems (human platelets and rat phaeochromocytoma cells) have shown that hyperforin increases both intracellular sodium ion and calcium ion concentrations, and that this is mediated through activation by hyperforin of nonselective cation channels.(47)

Hyperforin was shown to inhibit 5-HT reuptake in washed platelets but not in fresh platelet-rich plasma, suggesting that
plasma-protein binding could be a limiting factor for 5-HT uptake inhibition in vivo.(48)
A commercial extract of St John's wort has exhibited psychotropic and antidepressant activities in mice.(49) Pure hyperforin and hypericum extracts also demonstrated antidepressant activity in a despair behaviour test in rats.(37)
In other experimental models of depression, including acute and chronic forms of escape deficit induced by stressors, hypericum extract was shown to protect rats from the consequences of unavoidable stress.(50) In studies using the rat forced swimming test, an experimental model of depression, hypericum extracts induced a significant reduction in immobility.(51) Flavonoid fractions and flavonoids isolated from these fractions have also been reported to have antidepressant activity in the forced swimming test in rats.(52)
Cytotoxic and anticancer activities The findings of a substantial body of preclinical research have documented anticancer activites for H. perforatum preparations and their constituents. A methanolic extract of H. perforatum (containing hypericin 0.3% and hyperforin 3.8%) administered intraperitoneally (15 mg/kg body weight) ten days before implantation of PC- 3 human Caucasian prostate adenocarcinoma cells in nude mice
significantly reduced tumour growth and the number of regional lymph node metastases (p < 0.01 for both).(53) The same extract
at a concentration of 1.41 mg/mL also significantly inhibited the proliferation of PC-3 cells in vitro (IC50 = 0.42 mg/mL). Ethanolic extracts of H. perforatum from fresh and dried plant material (drug to extract ratio 1.3-1.5 : 1) inhibited the proliferation of human malignant cells (e.g. leukamia cell lines K562 and U937) in a concentration-dependent manner.(54) The extracts also induced apoptosis of glioblastoma LN229 cells. The observed effects were potentiated by light activation. In a similar series of experiments using extracts of H. perforatum containing 0.3% hypericins but differing concentrations of hyperforin (0.21%, 2.21% or 3.25% w/ w) and flavonoids (5.3% or 10% w/w), antiproliferative activity of the different extracts varied (GI50 values: 248.3 to 621.3 mg/mL and 378.2 to 911.7 mg/mL for K562 and U937 cell lines, respectively), indicating that the flavonoid constituents, as well as hypericin and hyperforin, contribute to the observed effects.(55) The possibility that constituents other than hypericin have cytotoxic and/or antiproliferative activity is supported by further in vitro work which showed that a methanolic extract of H. perforatum flowering parts inhibited growth of K562 cells and induced apoptosis to a greater extent than did hypericin alone.(56) In mice injected with murine and human cancer cell lines, seven days' pretreatment with hyperforin (as the stable dicyclohexylammonium salt) intraperitoneally reduced several markers of cancer infiltration and metastasis.(57) In rats given subcutaneous injections of MT-450 rat mammary carcinoma cells, treatment with hyperforin (100 mL of 2 mmol/L solution subcutaneously at the tumour site once daily) for two weeks starting 15 days after tumour injection inhibited tumour growth to a similar extent as did paclitaxel given according to the same dosage regimen.(58) There is evidence that the mechanism by which hyperforin induces
apoptosis involves the activation of caspases (inactive proen- zymes).(58, 59)
Numerous preclinical studies have established that hypericin is a photocytotoxic agent. Hypericin photosensitisation has been documented for various cancer cell lines in vitro(60–64) and in several in vivo experimental models of cancer.(61, 65) The
St John’s Wort |
553 |
|
photocytotoxic effects of hypericin towards human leukaemic |
|
|
HL-60 cells can be potientiated in vitro by co-incubation with |
|
|
acetazolamide(66) and quercetin.(67) Phototoxicity and induction of |
|
|
apoptosis also occur with pseudohypericin in vitro.(68) Hypericin |
|
|
photo-induced apoptosis may involve the tumour necrosis factor |
|
|
(TNF)-related apoptosis-inducing ligand,(69) activation of cas- |
|
|
pases, such as caspase-8,(69, 70) and inhibition of proteasome |
|
|
function (which is involved in caspase activation).(71) |
|
|
Further in vitro studies have added a layer of complexity to the |
|
|
above findings. Hypericin can induce apoptosis or necrosis, |
|
|
depending on the intracellular hypericin concentration and/or the |
|
|
light-activating dose.(72) Furthermore, exposure of U937 cells to |
|
|
hypericin and sub-lethal doses of light irradiation induced |
|
|
subsequent photoresistance with light doses which normally |
|
|
induced apoptosis.(73, 74) |
|
|
In an in vitro system involving the human cytochrome P450 |
|
|
enzyme CYP1A1, three commercially available H. perforatum |
|
|
extracts as well as several constituents of H. perforatum extracts |
|
|
(with the exception of rutin) inhibited the CYP1A1-catalysed |
|
|
epoxidation of ( )-trans-7,8-dihydro-7,8-dihydroxy-benzo(a)pyr- |
|
|
ene, the reaction which leads to formation of the carcinogenic |
|
|
product diolepoxide 2.(75) |
|
|
In vitro cytotoxicity against human colon carcinoma cells (CO |
|
|
115) has been described for hyperforin-related constituents |
|
|
isolated from Hypericum calycinum and Hypericum revolu- |
|
|
tum.(76) |
|
|
Antimicrobial activity Extracts of H. perforatum aerial parts |
|
|
have antibacterial activity against Gram-positive bacteria, parti- |
|
|
cularly Bacillus subtilis and B. cereus, but not Gram-negative |
|
|
bacteria and yeasts, according to the findings of a series of in vitro |
|
|
assays.(77) |
|
|
A leaf extract of H. perforatum has been documented as |
|
|
enhancing the immunity of mice towards Staphylococcus aureus |
|
|
and Bordetella pertussis.(78) Hyperforin has antibacterial activity |
|
|
against S. aureus,(9) multi-drug resistant S. aureus and Gram- |
|
|
positive bacteria, including Streptococcus pyogenes and Coryne- |
|
|
bacterium diphtheriae.(79) However, the antibacterial effects of |
|
|
hyperforin are only observed at high concentrations.(80, 81) Other |
|
|
experiments have shown that S. aureus is able to acquire resistance |
|
|
to hyperforin, but that this does not occur with hyperforin |
|
|
concentrations similar to those found in patients treated with H. |
|
|
perforatum extracts for depression.(82) Hyperforin did not exhibit |
|
|
any growth inhibitory effect against Gram-negative bacteria, such |
|
|
as Enterococcus faecalis, Escherichia coli and Pseudomonas |
S |
|
aeruginosa or against Candida albicans.(79) Other antibacterial |
||
constituents (imanine and novoimanine) have been isolated from |
|
|
St John's wort.(83, 84) |
|
|
Several other species of Hypericum have been shown to have |
|
|
antimicrobial activity.(85, 86) In disc-diffusion assays, 33 |
of 34 |
|
chloroform extracts of Hypericum species (not including H. perforatum) showed substantial activity against a clinical isolate of methicillin-resistant Staphylococcus aureus.(86)
Antiviral activity Flavonoid and catechin-containing fractions have exhibited antiviral activity, inhibiting the influenza virus by 83–100%.(87) Hypericin and pseudohypericin have been reported to inhibit several encapsulated viruses in vitro, including herpes simplex types 1 and 2,(88, 89) varicella-zoster virus(90) and human immunodeficiency virus type 1 (HIV-1).(91–94) Hypericin has also been reported to inactivate murine cytomegalovirus (MCMV) and Sindbis virus.(94) The antiviral activity of hypericin appears to involve a photoactivation process.(94, G1) An extract of a St John's wort product (5–50 mL/mL; no further details provided) and pure

554 St John’s Wort
hypericin (5–20 mmol/L) inhibited UV-induced HIV gene expression in HeLa cells in a concentration-dependent manner, whereas
hypericin without UV-induced HIV gene activation had no effect on HIV gene expression.(95)
Other effects In vitro studies using a hamster vas deferens smooth muscle cell line demonstrated that hyperforin induces the release of calcium ions from mitochondrial or other sources followed by activation of cellular metabolism.(96) It is not known whether this activity contributes to the antidepressant effects of hyperforin.
Oral administration of a single dose of St John's wort (100, 200, 400, 600 or 800 mg/kg) to two strains of alcohol-preferring rats significantly reduced alcohol intake in both strains.(97) In another study in experimental alcoholism, acute intraperitoneal administration of St John's wort (10–40 mg/kg), fluoxetine (1–10 mg/kg) and imipramine (3–30 mg/kg) reduced alcohol intake in a dosedependent manner in a 12-hour, limited access, two-bottle choice (ethanol/water) procedure.(51) In alcohol-preferring mice, the dose (5 mg/kg administered orally by gavage) of a hyperforin-rich carbon-dioxide extract of H. perforatum required to reduce the intake of 10% ethanol to a statistically significant extent was 125fold lower than that required with a crude methanolic extract (625 mg/kg) with negligible hyperforin content administered by the same route.(98) In mice, oral administration of a Hypericum perforatum extract (Ph-50) attenuated nicotine withdrawal symptoms.(99) Depression, alcoholism and smoking are thought to have some neurochemical similarities, such as low brain serotonin concentrations.(100, 101)
An extract of St John's wort was found to suppress
inflammation and leukocyte infiltration induced by carrageenan and prostaglandin E1 (PGE1) in mice.(102) In vitro, hypericin has
been shown to inhibit tumour necrosis factor-induced activation of the transcription factor NF-kB,(103) specific growth factorregulated protein kinases(104–106) and the release of arachidonic acid and leukotriene B4.(107) In a rabbit model of proliferative vitreoretinopathy (PVR), intravitreal injection of hypericin 0.1 mL (10 or 100 mmol/L, but not 1 mmol/L) inhibited the progression of PVR when compared with severity in control eyes five days after hypericin administration.(108) It was suggested that, as protein kinase C is important in the cellular reactions occurring in PVR, modulation of protein kinase C by hypericin may be a factor in this system. Hypericin and pseudohypericin have been reported to inhibit 12-lipoxygenase activity; the products of lipoxygenase-
Scatalysed reactions, include leukotrienes.(109) Hyperforin has also been shown to inhibit the activity of enzymes involved in
inflammatory pathways: in vitro, hyperforin inhibited 5-lipox- ygenase and cyclooxygenase 1, but not cyclooxygenase 2.(110)
Antioxidant properties have been reported for H. perforatum following in vitro experiments. St John's wort products (Nature's Plus and Movana, respectively) obtained in the USA and labelled as being hypericin- (0.3–0.5%) and hyperforin-standardised (minimum 3%) attenuated superoxide production in an inverse
concentration-dependent manner in a cell-free system and in an experimental model using human vascular tissue.(111) Hyperforin isolated from H. perforatum reduced radical formation by polymorphonuclear cells from healthy human donors after
stimulation with N-formyl-methionyl-leucyl-phenylalanine (IC50 = 1.8 mmol/L).(112) However, in other systems, hyperforin did not
exhibit any free-radical scavenging activity against 2,2-diphenyl-1- picrylhydrazyl and was inactive in an enzymatic assay based on oxygen radical production by horseradish peroxidase in the presence of hydrogen peroxide.
Imanine was reported to reduce blood pressure and increase the frequency and depth of breathing following intravenous administration (50 mg/kg) to rabbits.(83) A study of the vasoconstrictor action of water-soluble imanine and imanine on the isolated rabbit ear indicated that their hypotensive action was not due to a direct effect on the vasculature.(83) When perfused through the isolated frog heart, both water-soluble imanine and imanine were found to cause cardiac systolic arrest at a dilution of 1 10 5.(83) Proanthocyanidin-containing fractions isolated from St John's wort have been reported to inhibit contractions of the isolated
guinea-pig heart induced by histamine, PGF2a and potassium chloride.(113)
A tonus-raising effect on isolated guinea-pig and rabbit uteri has been documented for a crude aqueous extract.(114) Of the
group of plants investigated, St John's wort was reported to exhibit the weakest uterotonic activity.
A dried methanol extract of St John's wort (no further details provided) protected human neuroblastoma cells against hydrogen peroxide induced apoptosis in vitro.(115)
Analgesic activity in mice has been reported for a total flavonoid fraction of H. perforatum;(116) the active principle was stated to be of the quercetin type.
Tannins isolated from St John's wort are stated to have mild
astringent activity.(117) The anthraquinone derivatives documented for St John's wort do not possess any purgative action.(G62)
Clinical studies
Clinical trials with extracts of St John's wort have focused mainly on its effects in patients with depression, although there have been several studies exploring its use in other conditions, including
seasonal affective disorder, chronic fatigue and premenstrual syndrome.(118)
Pharmacodynamics Initially, hypericin was thought to be responsible for the antidepressant activity of St John's wort,
although, more recently, experimental(36, 37) and clinical evidence(119) has emerged to indicate that hyperforin is one of the
major constituents required for antidepressant activity.
The precise mechanism of action of St John's wort's antidepressant effect remains unclear (see Pharmacological Actions, In vitro and animal studies).
In a randomised, double-blind, placebo-controlled, crossover study involving 16 healthy volunteers who received St John's wort extract (Neuroplant) 300 mg three times daily for seven days, St John's wort extract did not influence plasma norepinephrine (noradrenaline) concentrations, but significantly increased plasma dihydroxyphenylacetic acid concentrations (the main metabolite of dopamine), compared with placebo (p = 0.013).(120)
Studies in humans have reported conflicting results with respect to the effects of St John's wort extracts on endocrinological parameters. A double-blind, placebo-controlled, crossover study in 12 healthy male volunteers investigated the effects of a single dose of St John's wort extract (LI 160) (2700 mg, 9 300-mg tablets standardised to 0.3% hypericin) on plasma concentrations of growth hormone, prolactin and cortisol.(121) A significant increase in plasma growth hormone concentration and a significant decrease in plasma prolactin concentration were observed following St John's wort administration relative to placebo administration. Plasma cortisol concentrations were unchanged. These findings suggest that this dose of St John's wort extract may increase aspects of brain dopamine function in humans, although further studies are required to confirm this, assess dose–response relationships and determine whether there is

evidence for effects on dopaminergic systems in patients with depression treated with St John's wort.(121) Another study, which used a randomised, three-way, crossover design, investigated the effects of a single dose of St John's wort extract (LI 160S) (600 or 300 mg) or placebo on hormone concentrations in 12 healthy male volunteers.(122) Compared with placebo, St John's wort extract (600 mg) increased cortisol secretion between 30 and 90 minutes after dosing, indicating an influence of St John's wort on certain CNS neurotransmitters. There was no difference between the three groups with regard to adrenocorticotrophic hormone (ACTH), growth hormone and prolactin secretion.(122)
By contrast, in a randomised, single-blind, placebo-controlled, crossover study involving 12 healthy male volunteers, mean serum ACTH concentrations, but not cortisol, growth hormone and prolactin concentrations, were significantly increased following oral administration of a St John's wort extract (WS-5570) at doses of 600, 900 and 1200 mg/day on four different days (p < 0.05 versus placebo). However, there were no significant differences in
ACTH concentrations between groups when a statistical adjustment (Bonferroni correction) was made for post-hoc tests.(123)
Differences in the findings of this study, compared with previous
work, may be due to differences between doses, products tested and their bioavailabilities.(123)
Therapeutic effects Depression There are now over forty clinical trials of H. perforatum preparations involving patients with different types of depression, and many of these trials have been included in systematic reviews.
A Cochrane systematic review included 37 randomised, double-blind, controlled clinical trials of monopreparations of H. perforatum involving a total of 4925 patients with depressive disorders.(24) Of these, 26 trials were placebocontrolled, 14 compared H. perforatum preparations with standard antidepressants (imipramine 50 to 150 mg daily, four trials; fluoxetine 20 to 40 mg daily, four trials; sertraline 75 to 150 mg daily, three trials; amitriptyline 30 or 75 mg daily, two trials; maprotiline 75 mg daily, one trial) and three of these studies(124–126) also included a placebo control group. Trials involved a variety of H. perforatum preparations administered at doses of 240 to 1800 mg extract daily. The treatment period was typically four to six weeks (29 trials) although administration periods ranged from four to 12 weeks overall. The most frequently investigated product was LI-160 (Lichtwer Pharma, Germany). Overall, 24 trials involved only patients with major depression. In most trials (n = 35), the Hamilton Rating Scale for Depression (HAMD) was the instrument used to assess outcomes, and the methodological quality of the majority of
the included studies was considered to be 'reasonable to good'.(24)
Of the 26 trials (involving 3320 participants) comparing H. perforatum preparations with placebo, 23 provided data which were eligible for meta-analysis. For these studies, the results indicated that H. perforatum preparations were more effective than placebo (response rate ratio (RRR), 95% confidence interval (CI): 1.55, 1.42–1.70), although when the analysis was limited to larger (i.e. more precise) trials in patients with major depression only, the results showed a small benefit for H. perforatum over placebo (RRR, 95% CI: 1.15, 1.02–1.129).(24) Similarly, meta-analysis of data from the 23 placebo-controlled trials which used HAMD scores to assess outcomes indicated that H. perforatum preparations were more effective than placebo (weighted mean difference (WMD), 95% CI: 3.25,3.74 to 2.77), but when the analysis was restricted to the 12
St John’s Wort |
555 |
|
such trials involving only patients with major depression, the |
|
|
effect was less marked (WMD, 95% CI: 2.48, 3.06 to |
|
|
1.89). |
|
|
Meta-analysis of data from the 14 trials (involving 2283 |
|
|
participants) comparing H. perforatum with standard anti- |
|
|
depressant agents indicated that H. perforatum preparations |
|
|
had similar effects to those of standard antidepressants (RRR, |
|
|
95% CI: 1.01, 0.93 to 1.10 and WMD, 95% CI: 0.06, 0.64 |
|
|
to 0.51 for trials providing response rate data (13 trials) and |
|
|
HAMD scores (11 trials), respectively).(24) Results were similar |
|
|
for sub-analysis of the trials comparing H. perforatum |
|
|
preparations with older antidepressants and comparisons |
|
|
with newer antidepressants (selective serotonin reuptake |
|
|
inhibitors). |
|
|
The conclusions drawn from this work were that the |
|
|
evidence is inconsistent and complex. H. perforatum prepara- |
|
|
tions and standard antidepressant agents appear to show |
|
|
similar effects, whereas H. perforatum preparations have only |
|
|
small benefits over placebo in patients with major depression; |
|
|
in older studies in patients with mild-to-moderate depression, |
|
|
H. perforatum preparations appear to be of more benefit than |
|
|
placebo.(24) An important point is that there is heterogeneity |
|
|
not only among the trials and their results, but also among the |
|
|
different manufacturers' products tested. Products are not |
|
|
necessarily equally effective, and the results of the analyses |
|
|
above should not be extrapolated to other H. perforatum |
|
|
preparations, which may differ considerably in their pharma- |
|
|
ceutical quality (see Quality of plant material and commercial |
|
|
products). |
|
|
One of the trials included in the systematic review was a |
|
|
randomised, double-blind, placebo-controlled, multicentre |
|
|
study comparing an extract of H. perforatum (LI-160, |
|
|
standardised for hypericin 0.12–0.28%) 900 mg daily with the |
|
|
SSRI sertraline (which is authorised for the treatment of |
|
|
depression) at a dose of 50 mg daily in 340 patients with major |
|
|
depressive disorder. The trial was funded by the National |
|
|
Center for Complementary and Alternative Medicine and the |
|
|
National Institute of Mental Health, USA, and was designed to |
|
|
assess whether or not H. perforatum extract was superior to |
|
|
placebo after 8 weeks' treatment.(126) However, at the end of |
|
|
the study, there were no statistically significant differences in |
|
|
the two primary outcome measures (changes in HAMD scores |
|
|
and response rate) not only between H. perforatum extract and |
|
|
placebo, but also between sertraline and placebo. Thus, this |
S |
|
trial was criticised for lacking the sensitivity at least to detect |
||
the effectiveness of a treatment known to be effective (i.e. |
||
sertraline). |
|
|
A further issue which arose subsequently relates to |
|
|
adherence to treatment among participants randomised to |
|
|
the H. perforatum group. Analysis of blood samples available |
|
|
for 97 of the 113 H. perforatum recipients revealed that 80 |
|
|
(82%) had detectable concentrations of hyperforin in at least |
|
|
one sample, whereas 17 (17%) had no detectable hyperforin in |
|
|
any of their samples.(127) The sensitivity threshold of the assay |
|
|
was hyperforin 10 ng/mL. Furthermore, of 104 of |
the 116 |
|
participants randomised to placebo for whom blood samples were available, 18 (17%) had detectable concentrations of hyperforin in at least one sample. By contrast, sertraline and/or N-desmethylsertraline were detected in at least one sample for all sertraline recipients for whom blood samples were available (91 of 111). Reanalysis of the efficacy data using only data from participants with plasma hyperforin concentrations consistent

556 St John’s Wort
with their treatment allocation did not change the initial findings.(127)
A randomised, double-blind, multicentre trial comparing the effects of a hydroalcoholic extract of H. perforatum herb (WS5570, drug to extract ratio 3–7 : 1, standardised for hyperforin 3–6% and hypericin 0.12–0.28%; Schwabe Pharmaceuticals) with those of paroxetine in the acute treatment of moderate to severe depression has been published since the revised Cochrane review was completed. In the study, after a placebo run-in phase, 251 participants with acute major depression received H. perforatum extract 300 mg three times daily (increased to 1800 mg daily in non-responders), or paroxetine
20 mg daily (40 mg daily for non-responders), for six weeks.(128) At the end of the study, the H. perforatum extract
was reported to be at least as effective as paroxetine in reducing symptoms of moderate to severe depression (mean (standard deviation) reduction in HAMD scores from baseline values: 14.4 (8.8) and 11.4 (8.6) for H. perforatum and paroxetine, respectively).
The review described above(24) was an update of a previous Cochrane systematic review and meta-analysis of 27 randomised controlled trials of H. perforatum preparations in depressive disorders.(129) The updated review had stricter and tighter inclusion and exclusion criteria for trials – only trials which explicitly stated that the method of treatment allocation was random and which used a double-blind design were included, and trials investigating H. perforatum for prevention of depression, those using combination preparations containing H. perforatum, comparing H. perforatum with drugs not explicitly recommended as antidepressant agents, measuring
only physiological parameters and those with a treatment period of less than four weeks were excluded.(24) Thus, the
previous Cochrane review included seven trials which were excluded from the updated review.
The results of the earlier meta-analysis showed that H. perforatum preparations were significantly superior to placebo in the short-term treatment of mild to moderately severe depressive disorders (rate ratio 2.47 and 95% confidence interval (95% CI) 1.69–3.61). and as effective as conventional antidepressant agents (single preparations, rate ratio 1.01 and 95% CI 0.87–1.16), although for several reasons – for example, the use of low doses of conventional antidepressants and the trials involving small numbers of patients – this evidence was considered inadequate for establishing whether H. perforatum
Spreparations were as effective as conventional antidepressant drugs.(129) Further studies comparing St John's wort preparations with standard antidepressant agents in well-defined patient groups and over longer periods were considered
necessary.(129)
Another earlier meta-analysis employed tighter inclusion criteria for trials in an effort to increase the validity of the analysis.(130) It included only randomised, blinded, controlled trials of St John's wort as a single preparation, which involved patients with depressive disorders as defined by the standard criteria ICD-10 (International Statistical Classification of Diseases and Related Health Problems), DSM-IIIR (Diagnostic and Statistical Manual) or DSM-IV and which used the Hamilton Depression (HAMD) Scale for measuring clinical outcomes. Six such trials involving 651 patients with mainly mild to moderately severe depressive disorders were included; two trials were placebo controlled and four compared St John's wort with standard antidepressants. The studies lasted for 4–6 weeks and the doses of St John's wort extract ranged from 200
to 900 mg daily; the range for total hypericin administered was 0.75–2.7 mg daily.
This meta-analysis showed that the response rate for St John's wort was significantly greater than that for placebo (73.2% versus 37.9%, respectively, relative risk 1.48 and 95% CI 1.03–1.92) and similar to that observed with tricyclic antidepressants (64% versus 6.4% for St John's wort and
tricyclic antidepressants, respectively, relative risk 1.11 and 95% CI 0.92–1.29).(130) Despite the stringent inclusion criteria
for trials in this meta-analysis, it was concluded that further studies are required in order to address methodological problems before it can be concluded that St John's wort is an effective antidepressant.(130)
A systematic review of large-scale observational studies of H. perforatum extracts in patients with depressive disorders is also available. The review included 16 non-randomised studies (involving a total of 34 804 patients) each involving at least 100 participants with depressive disorders who were treated with H. perforatum preparations for at least four weeks.(131) Fifteen of the studies reported physician-assessed response rates, and these ranged from 65% to 100% for short-term studies (four to around six weeks' treatment; 13 studies) and were 60% and 69% for the two long-term studies (52 weeks' treatment). Patient-assessed response rates ranged from 63% to 98% (ten studies). These results suggest that the H. perforatum extracts assessed are effective for mild and moderately severe depressive disorders, although this conclusion cannot be definitive since the studies did not include random allocation to treatment and many had other methodological limitations.(131) Furthermore, the studies included in the review assessed the effects of 12 different H. perforatum products, with some differences in how they were standardised, administered at doses ranging from 360 to 1200 mg extract daily. Thus, the results cannot be extrapolated directly to other H. perforatum preparations with a different phytochemical profile.
One of the studies included in the review assessed the effects of an H. perforatum preparation in children aged under 12 years with symptoms of depression and psychovegetative disturbances. This study reported the highest physicianand
patients-assessed response rates (100% and 98%, respectively).(132) Other open, uncontrolled studies(133) have explored
the effects of H. perforatum preparations in children and adolescents, although the efficacy of such preparations in these patient groups requires testing in randomised controlled trials.
In a dose-ranging trial involving 348 patients with mild to moderate depression according to ICD-10 criteria, patients were randomised to receive St John's wort extract three times
daily equivalent to either 1 mg (n = 119), 0.33 mg (n = 115) or 0.17 mg (n = 114) hypericin for six weeks.(134) At the end of the
treatment period, there was a significant reduction in HAMD scores compared with baseline values. The response rates (according to recognised criteria) were 68%, 65% and 62% for 1, 0.33 and 0.17 mg hypericin, respectively; the differences between groups were not statistically significant. Thus, the study showed that there was no dose-dependent effect of hypericin in St John's wort extracts.
Smoking cessation A preliminary, uncontrolled study has assessed the effects of a methanol (80%) extract of H. perforatum herb (LI-160, standardised for hypericin 900 mg and a minimum of 2% hyperforin; drug to extract ratio 3–6 : 1) as an aid to motivational/behavioural support in adult smokers who wish to stop smoking. The rationale for investigating H.

perforatum in this indication is that there is an association between smoking and depression: nicotine may act as an antidepressant in some smokers, and depression can be precipitated by nicotine withdrawal, i.e. smoking cessation.(101) In the study, point prevalence and continuous abstinence rates were both 18% at 3 months, and 0% at 12 months (intention- to-treat analysis); these rates compare poorly with response rates in placebo groups in controlled clinical studies of other smoking cessation interventions.(135)
Seasonal affective disorder The effects of St John's wort extracts have been investigated in studies involving subjects with seasonal affective disorder (SAD),(136, 137) although as yet there have not been any trials that have included a placebo control group. Twenty individuals with SAD were randomised to receive St John's wort (LI 160) (300 mg) three times daily (equivalent to 0.9 mg hypericin) with or without bright light therapy.(136) After four weeks, there were significant reductions in HAMD scores in both groups compared with baseline values and there were no statistically significant differences between groups. Another study evaluated data from individuals with mild to moderate SAD who had used St John's wort (300 mg)
three times daily (equivalent to 0.9 mg hypericin) with (n = 133) or without light therapy (n = 168) for eight weeks.(137)
The study was not randomised and involved data collection by postal questionnaires. Data from 301 returned questionnaires were suitable for analysis. Significant reductions in the mean SAD scores were observed in both groups compared with baseline values; the differences in the SAD scores between groups were statistically non-significant.
Antiviral activity Antiviral activity has been reported for
hypericin |
against |
human |
immunodeficiency |
virus |
(HIV).(138, 139) Several |
uncontrolled studies in HIV-positive |
|||
patients who received St John's wort extract have reported
immunologic and clinical benefits, including increases in CD4 cell counts in some patients.(140, 141) In a phase I, dose-
escalating study, 30 HIV-positive patients with CD4 cell counts <350 cells/mm3 received intravenous synthetic hypericin twice
weekly (0.25 or 0.5 mg/kg body weight), three times weekly (0.25 mg/kg) or oral hypericin daily (0.5 mg/kg).(142) Sixteen
patients discontinued treatment early because of toxic effects, and phototoxicity in several other patients prevented completion of dose escalation. Antiretroviral activity as assessed by significant changes in HIV p24 antigen level, HIV titre, HIV RNA copies and CD4 cell counts was not observed.
In contrast, in a phase-I dose-escalation study involving 19 patients with hepatitis C virus (HCV) infection who received hypericin administered orally at doses of 0.05 and 0.10 mg/kg daily for eight weeks, there was no evidence of antiviral activity
as determined by median changes in HCV RNA concentrations in plasma.(143)
Other studies The potential for the use of St John's wort in 20 individuals presenting with fatigue(144) and in 19 women with self-reported premenstrual syndrome(145) has also been
explored in uncontrolled pilot studies. Significant improvements in perceived fatigue and in symptoms of depression and anxiety were seen after six weeks' treatment with St John's wort (equivalent to 0.9 mg hypericin daily) compared with baseline values(144) and in overall premenstrual syndrome
scores after treatment with St John's wort (equivalent to 0.9 mg hypericin daily) for two menstrual cycles.(145) Thus, there is
St John’s Wort |
557 |
|
scope for conducting randomised controlled trials of St John's |
|
|
wort in these conditions.(144, 145) |
|
|
A randomised, double-blind, placebo-controlled trial |
|
|
assessed the effects of an 80% methanol extract of St John's |
|
|
wort (LI-160; containing 300 mg extract, drug to extract ratio |
|
|
4–7 : 1) in 175 patients with somatoform disorders (usually |
|
|
characterised by chronic multiple physical symptoms not |
|
|
explained by underlying organic pathology). Participants |
|
|
received St John's wort extract 300 mg twice daily (n = 87), |
|
|
or placebo (n = 88), for six weeks. At the end of the study, |
|
|
according to an intention-to-treat analysis, St John's wort |
|
|
recipients, compared with placebo recipients, showed statisti- |
|
|
cally significant improvements on the six individual variables |
|
|
comprising the primary efficacy analysis and in the combined |
|
|
score (p < 0.0001 for all comparisons).(146) |
|
|
Another randomised, double-blind, parallel-group trial |
|
|
investigated the effects of LI-160 300 mg extract twice daily |
|
|
(increased in increments to a maximum of 1800 mg extract |
|
|
daily at the physician's discretion), or placebo, for 12 weeks in |
|
|
41 individuals with social phobia (social anxiety disorder). At |
|
|
the end of the study, there was no statistically significant |
|
|
difference between the two groups in Liebowitz Social Anxiety |
|
|
Scale scores, the primary outcome measure (p = 0.79).(147) |
|
|
In a randomised, double-blind, placebo-controlled trial, 179 |
|
|
women with menopause-related psychovegetative symptoms |
|
|
received a combination preparation of St John's wort and black |
|
|
cohosh (Cimicifuga racemosa) or placebo for six weeks.(148) |
|
|
The results indicated that the combination product had a |
|
|
significantly greater effect on the symptoms than did placebo. |
|
|
A randomised, double-blind, phase I study involving 55 |
|
|
healthy volunteers who received St John's wort (900 mg) daily |
|
|
(containing 0.5% hyperforin), St John's wort (900 mg) daily |
|
|
(containing 5.0% hyperforin) or placebo for eight days |
|
|
investigated the effects on quantitative electroencephalogram |
|
|
as an indicator of drug-induced pharmacological action.(149) |
|
|
Reproducible central pharmacodynamic effects were apparent |
|
|
in both groups of St John's wort recipients compared with |
|
|
placebo recipients. The effects were greater in subjects who |
|
|
received extract containing 5.0% hyperforin than in those who |
|
|
received extract containing 0.5% hyperforin. |
|
|
Placebo-controlled, crossover studies investigating the effects |
|
|
of St John's wort (0.9 and 1.8 mg) on the sleep polysomnogram |
|
|
of healthy subjects reported that both doses of St John's wort |
|
|
significantly increased rapid eye movement (REM) sleep |
S |
|
latency compared with placebo, but had no effect on REM |
||
sleep duration or other parameters of sleep architecture.(150) |
||
In a randomised, double-blind, placebo-controlled trial involving 23 overweight but otherwise healthy adults, subjects who received treatment with St John's wort (900 mg) daily, Citrus aurantium extract (975 mg) daily and caffeine (528 mg) daily lost significantly more body weight than did subjects in the placebo and no-treatment control groups.(151)
A placebo-controlled, crossover study in 19 healthy volunteers who received St John's wort for 15 days either alone or in combination with ethanol (to achieve a blood alcohol concentration of 0.05%) reported that there were no differences between the two groups in sense of well-being or adverse events.(152)
A randomised, double-blind, placebo-controlled, six-week trial involving 72 long-distance runners and triathletes reported significant improvements in endurance capacity in subjects who
received vitamin E with St John's wort compared with subjects who received vitamin E alone or placebo.(153)

558 St John’s Wort
In a randomised, double-blind trial involving 21 patients with symmetrical mild to moderate atopic dermatitis, participants used a topical preparation of H. perforatum (cream containing 5% of a carbon-dioxide extract; drug to extract ratio 20–25 : 1, containing total 9.9% hyperforins) with a final hyperforin content of 1.5%, or placebo, twice daily for four weeks.(154) Treatment was randomly allocated to be applied to the left or right side of the body. At the end of the study, the H. perforatum preparation was found to be superior to placebo with respect to the primary outcome measure (clinical intensity of skin lesions; p < 0.022 for H. perforatum versus placebo).
In a randomised, double-blind, crossover trial involving 54 diabetic and non-diabetic patients with polyneuropathy, the analgesic effects of an H. perforatum preparation (Calmigen, Sanopharm; each tablet containing hypericin 900 mg, no further
details of preparation provided) one tablet three times daily were compared with those of placebo.(155) The rationale for
investigating the effects of H. perforatum on pain was centred around evidence that the antidepressant effect of H. perforatum preparations is due to effects on monoaminergic systems, and that effects on such systems may be the mechanism of action of agents such as tricyclic antidepressants currently used in painful polyneuropathy. In the study, patients' daily ratings of pain using numeric rating scales were used as the primary outcome measure. At the end of the treatment period, there were no statistically significant differences in total pain score between the H. perforatum and placebo groups (14 and 15, respectively; p = 0.05), in individual pain rating scales (p = 0.09
to 0.33), or in participants' evaluations of pain relief (p = 0.07).(155)
In a double-blind, controlled, crossover study, 12 healthy volunteers aged 18 to 54 years received tablets containing an extract of H. perforatum (Hyperiforte, each tablet contained 300 mg extract standardised for hyperforin 3–5% and hypericin 0.3%). Participants received placebo, and three and six tablets of the extract as a single dose in a random order and undertook a battery of memory tests before and after each administration. The results indicated that the H. perforatum extract did not have nootropic effects,(156) although as a sample size calculation does not appear to have been carried out, it is possible that the study did not have adequate statistical power to detect any differences.
A preliminary study found that a mixture of oils extracted
Sfrom H. perforatum and Calendula arvensis reduced the surface area of wounds in women who had undergone
Caesarean section during childbirth, when compared with control (wheatgerm oil).(157) However, the study did not involve random allocation to treatment and was not blinded, so the findings cannot be attributed definitively to the intervention.
The use of intravesical instillation of hypericin as a photosensitiser in human bladders together with blue light irradiation and fluorescence detection has been described as a diagnostic tool for the detection of bladder carcinoma. The
technique had 98.5% specificity in detecting carcinoma in situ and dysplasia, and had a sensitivity of 93%.(158, 159)
Pharmacokinetics Detailed pharmacokinetic studies have been carried out with the hypericin-standardised St John's wort extract LI 160 and with certain other H. perforatum extracts.(1, G1) Administration of single oral doses of LI 160 (300, 900 and 1800 mg) to healthy male volunteers resulted in peak plasma hypericin concentrations of 1.5, 7.5 and 14.2 ng/mL for the three
doses, respectively. Peak plasma concentrations were seen with hypericin after 2.0–2.6 hours and with pseudohypericin after 0.4– 0.6 hours. The elimination half-life of hypericin was between 24.8
and 26.5 hours. Repeated doses of LI 160 (300 mg) three times daily resulted in steady-state concentrations after four days.(160)
Oral administration of the St John's wort extract WS 5572 (300 mg, equivalent to 14.8 mg hyperforin) resulted in peak plasma concentrations of 150 ng/mL being reached 3.5 hours after administration.(161) The elimination half-life was 9 hours. Following repeated doses of 300 mg three times daily, the estimated steady-state plasma hyperforin concentrations were 100 ng/mL.
In open trials involving 18 healthy male volunteers, following oral administration of a single tablet of a dry extract of H. perforatum (STW-3, containing 612 mg extract equivalent to hypericin 600 mg, pseudohypericin 1200 mg, hyperforin 13.5 mg, flavonoids 73.2 mg; drug to extract ratio 5–8 : 1), pharmacokinetic parameters for hypericin, pseudohypericin and hyperforin, respectively, were: maximum plasma concentration, 3.14, 8.5
and 83.5 ng/mL; time to maximum concentration, 8.1, 3.0 and 4.4 hours; elimination half-life, 23.8, 25.4 and 19.6 hours.(162) The
flavonoid compounds quercetin and isorhamnetin showed two peaks of maximum plasma concentration, separated by about four hours (quercetin: 47.7 ng/mL by 1.2 hours and 43.8 ng/mL by 5.5 hours; isorhamnetin: 7.6 ng/mL by 1.5 hours and 9.0 ng/mL by 6.4 hours). The elimination half-life for these constituents was 4.2 and 4.5 hours for quercetin and isorhamnetin, respectively.
Pharmacokinetic parameters following multiple dosing (STW-3 once daily for 14 days) were similar.(162)
In an open, randomised, two-way, crossover study involving 12 healthy volunteers, the bioavailability of hyperforin after a single oral administration of a softgel capsule containing H. perforatum dry extract 300 mg (containing 0.3% hypericin and 5% hyperforin) was found to be superior to that following a single oral administration of a hard gelatin capsule containing the same extract.(163) The mean (standard deviation) peak plasma hyperforin concentrations were 168.4 (57.8) and 84.3 (33.5) ng/mL following administration of the softgel and hard gelatin capsules, respectively, although a p value was not reported. Hypericin, however, was not detectable in almost half of the participants for each dosage form. In nine male patients with superficial transition cell carcinoma of the bladder, intravesical instillation to the bladder of 40 mL of a 8 mmol/L hypericin solution for 2 to 3 hours followed by photodynamic diagnosis of bladder tumours, plasma concentrations of hypericin in samples taken one hour after the
end of the instillation were below the detection limit (<6 nmol/
L).(164)
A method for the simultaneous determination of hypericin and hyperforin in human plasma using liquid chromatography and tandem mass spectrometry (LC–MS–MS) has been developed and validated.(165) Using the method, the limits of quantification for hypericin and hyperforin were 0.05 ng/mL and 0.035 ng/mL, respectively. These low limits may allow detection of ingestion of hypericin and hyperforin for up to several days after discontinuation of treatment.
Side-effects, Toxicity
Clinical data
Data relating to the frequency and type of adverse effects associated with treatment with St John's wort extracts are available from randomised controlled trials, systematic reviews

and meta-analyses of such trials, and from post-marketing surveillance and other observational studies. Collectively, the data indicate that certain St John's wort extracts are well-tolerated
when taken at recommended doses for shorter periods of time (around eight weeks).(131, 166) Data from the small number of
longer-term (one year) studies support the tolerability of certain St John's wort extracts, although further investigation of long-term use is warranted. Adverse events/effects reported are generally mild and most commonly gastrointestinal symptoms. These observations, however, are based on data collected in the settings of formal randomised or observational studies, usually where H. perforatum has been prescribed under the supervision of a physician, not taken as self-treatment.
A small number of studies has explored the effects of selftreatment with St John's wort products. In a cross-sectional study involving 452 members of a depression self-help group (response rate = 17%), 63 of the 452 respondents (28%) reported adverse effects, including psychological symptoms, allergic reactions and
visual disturbances, that they believed to be related to use of St John's wort.(167) The safety of St John's wort products taken as
self-treatment without supervision by a healthcare professional requires further study.
A Cochrane systematic review included 37 randomised, doubleblind, controlled clinical trials of monopreparations of H. perforatum and involving a total of 4925 patients with depressive disorders (see Therapeutic effects, Depression).(24) Of the 26 placebo-controlled trials included in the review, data for analysis of the number of participants withdrawing for any reason were available from 19 trials, for withdrawing due to adverse effects from nine trials and for numbers of patients reporting adverse effects from 16 trials. Compared with placebo recipients, slightly fewer H. perforatum recipients withdrew from trials for any reason (odds ratio, 95% CI: 0.82, 0.64–1.06), withdrew due to adverse effects (odds ratio, 95% CI: 0.61, 0.28–1.31), and reported adverse effects (odds ratio, 95% CI: 0.79, 0.61–1.03). Compared with SSRIs, H. perforatum extracts were associated with a slightly lower probability of withdrawing from the study due to adverse effects (odds ratio, 95% CI: 0.60, 0.31–1.15; data from six trials) and reporting of adverse effects (odds ratio, 95% CI: 0.75, 0.52– 1.08; data from five trials), whereas overall withdrawal rates were similar (odds ratio, 95% CI: 0.95, 0.65–1.40; data from six trials).(24) Compared with older antidepressant agents, H. perforatum extracts were associated with a lower probability of withdrawing for any reason (odds ratio, 95% CI: 0.65, 0.46–0.92; data from seven trials), withdrawing due to adverse effects (odds ratio, 95% CI: 0.25, 0.14–0.45; data from six trials) and of reporting adverse effects (odds ratio, 95% CI: 0.39, 0.31–0.50; data from seven trials). It is important to consider that there is qualitative and quantitative variation in the composition of different manufacturers' products and the results of the analyses above should not be extrapolated to other H. perforatum preparations.
The review described above(24) was an update, with tighter inclusion and exclusion criteria, of a previous Cochrane systematic review of 27 randomised controlled trials of H. perforatum preparations in depressive disorders.(129) The previous review reported that, in the trials comparing St John's wort with standard antidepressants, the proportions of patients reporting
side-effects were 26.3% and 44.7%, respectively (rate ratio 0.57 and 95% CI 0.4–0.69).(129) Another meta-analysis which employed
tight inclusion criteria reported that tricyclic antidepressants were associated with a higher proportion of side-effects than were St
St John’s Wort |
559 |
|
John's wort preparations (47% versus 26.4%, respectively, relative |
|
|
risk 1.72 and 95% CI 1.30–2.14).(130) |
|
|
A randomised, double-blind, multicentre trial comparing the |
|
|
effects of a hydroalcoholic extract of H. perforatum herb (WS- |
|
|
5570, drug to extract ratio 3–7 : 1, standardised for hyperforin 3– |
|
|
6% and hypericin 0.12–0.28%; Schwabe Pharmaceuticals) with |
|
|
those of paroxetine in the acute treatment of moderate to severe |
|
|
depression has been published since the revised Cochrane |
|
|
review(24) was completed. In the study, 251 participants with |
|
|
acute major depression received H. perforatum extract |
300 mg |
|
three times daily (increased to 1800 mg daily in non-responders), |
|
|
or paroxetine 20 mg daily (40 mg daily for non-responders), for six |
|
|
weeks.(128) During the study, 55% of H. perforatum recipients |
|
|
reported a total of 172 adverse events and 76% of paroxetine |
|
|
recipients reported a total of 269 adverse events, representing |
|
|
incidences of 0.035 and 0.060 adverse events per day of exposure |
|
|
for H. perforatum and paroxetine, respectively. Gastrointestinal |
|
|
adverse events were the most common adverse events reported for |
|
|
both groups. Data were not provided on numbers and types of |
|
|
adverse events considered to be related to treatment. |
|
|
A systematic review of large-scale observational studies of H. |
|
|
perforatum extracts in patients with depressive disorders also |
|
|
provides data on adverse events. The review included 16 non- |
|
|
randomised studies (involving a total of 34 804 patients) each |
|
|
involving at least 100 participants with depressive disorders who |
|
|
were treated with H. perforatum preparations for at least four |
|
|
weeks (see Therapeutic effects, Depression).(131) Overall, 13 |
|
|
studies reported data on proportions of participants withdrawing |
|
|
due to adverse events; these ranged from 0% to 2.8% in short- |
|
|
term studies and from 3.4% to 5.7% in longer-term (one year) |
|
|
studies. Twelve studies reported data on proportions of partici- |
|
|
pants with adverse events (range 0–49.3%) and 12 (not the same |
|
|
12 studies) reported data on proportions of participants with side- |
|
|
effects (range 0–5.9%). These data, however, should be inter- |
|
|
preted cautiously as most of the studies did not describe |
|
|
adequately how these data were collected, clear definitions for |
|
|
the terms adverse events and adverse effects were not always |
|
|
provided, and as no serious adverse events were reported among |
|
|
the 30 000 patients, this raises questions about the methods used |
|
|
to collect data.(131) The most frequently reported adverse events or |
|
|
side-effects were gastrointestinal symptoms, followed by increased |
|
|
sensitivity to light and other skin reactions. |
|
|
Photosensitivity Sensitivity to sunlight following the ingestion of |
S |
|
hypericum or hypericin, the photosensitising agent in H. |
||
perforatum,(168, G33) is known as hypericism. |
|
|
Several studies have explored the photosensitising potential of hypericin-containing H. perforatum extract following oral administration. In a double-blind, crossover, single-dose study involving 13 healthy volunteers who received placebo or St John's wort extract (LI 160) (900, 1800 and 3600 mg containing 0, 2.81, 5.62 and 11.25 mg total hypericin, respectively), no evidence of photosensitivity was observed with or without St John's wort
following skin irradiation with both UV-A and UV-B light 4 hours after dosing.(169) In a multiple-dose study in which 50 volunteers
received St John's wort (LI 160) (600 mg) three times daily (equivalent to 5.6 mg total hypericin daily) for 15 days, a moderate increase in UV-A sensitivity was observed.(169) However, the doses used were higher than those recommended therapeutically. In another single-dose study, administration of St John's wort (LI 160) (1800 mg, equivalent to 5.4 mg total hypericin) to 12 healthy volunteers resulted in a mean serum total hypericin concentration of 43 ng/mL and a mean skin blister fluid concentration of 5.3 ng/

560 St John’s Wort
mL.(170) After administration of St John's wort (300 mg) three times daily for seven days in order to achieve steady-state concentrations, the mean serum total hypericin concentration was 12.5 ng/mL and the mean skin blister fluid concentration was 2.8 ng/mL; these concentrations are below those estimated to be phototoxic.(170) Further randomised, controlled, single- (six or 12 tablets of LI-160, equivalent to 5.4 and 10.8 mg hypericins, respectively; n = 48) and multiple-dose studies (three tablets of LI160 daily, equivalent to 2.7 mg hypericins daily, for seven days; n = 24) found no statistically significant differences in minimum erythema threshold doses (of light irradiation), erythema index
and pigmentation index between treated and untreated skin areas (p > 0.05 for each).(171, 172)
Collectively, the evidence indicates that the threshold for phototoxicity of hypericin is between 100 and 1000 ng/mL.(171) Since serum and skin concentrations of hypericin after oral administration of recommended doses are below 100 ng/mL, photosensitivity seems unlikely. However, caution is advised as it is possible that there may be unusual absorption of hypericin in some individuals and, particularly in fair-skinned individuals and after extended periods of solar irradiation, there may be increased susceptibility to the photosensitising properties of hypericin.(171)
A study reported that HIV-positive patients treated with oral hypericin (0.05 mg/kg) for 28 days developed mild symptoms of photosensitivity on exposure to sunlight and that two patients
developed intolerable symptoms of photosensitivity when the dose was increased to 0.16 mg/kg.(173) In a dose-escalating study
involving 30 HIV-infected patients treated with oral (0.5 mg/kg daily) or intravenous hypericin (starting dosage 0.25 mg/kg twice or three times weekly), 16 patients discontinued treatment before completing eight weeks of therapy because of moderate or severe phototoxicity; severe cutaneous phototoxicity was observed in 11 out of 23 evaluable patients.(142) Other serious clinical or laboratory adverse events were infrequent: elevation of alkaline phosphatase and hepatic aminotransferase concentrations to more than five times normal values was noted in two and three patients, respectively.
The effects of topical application of H. perforatum extract on skin sensitivity have also been investigated. In a randomised controlled trial, 16 healthy volunteers with no history of skin disease or photosensitivity were treated with H. perforatum oil (containing hypericin 110 mg/mL) or H. perforatum ointment (containing an alcoholic H. perforatum extract with a final hypericin concentration of 30 mg/mL). The oil or ointment was
Sapplied to a two centimetre test area on one forearm of participants for 24 hours. Controls were an untreated test area of the same forearm, and the untreated other forearm. After removal of the oil or ointment, both forearms of participants
received increasing doses of solar simulated irradiation (24, 48, 96 and 144 J/cm2). No phototoxic reactions were observed in any of
the participants, and there was no change in the minimal erythema dose in either the oil or the ointment group,(174) although data to support the latter statement were not shown. There was a significant increase in the erythema index following treatment with H. perforatum oil (which contained the higher concentration of hypericin) but not after H. perforatum ointment (p < 0.01 and p > 0.05, respectively, compared with values for untreated skin), although this may not be clinically relevant since the minimal erythema dose did not change. The phototoxic potential of topical application of the H. perforatum preparations tested appears to be low, although caution is necessary, particularly as hypericin may penetrate more highly through broken or lesional skin, and there may be increased susceptibility
to the photosensitising properties of hypericin in fair-skinned individuals and after extended periods of solar irradiation.(174)
A case report describes a 45-year-old woman who developed
blisters on two occasions at the treatment site following laser therapy to her legs for multiple solar keratoses.(175) It was stated
that the patient revealed that she was taking St John's wort, although a sample of the product was not obtained and, therefore, no analysis was undertaken. The blisters resolved and, after stopping treatment with St John's wort, the patient underwent a further session of laser treatment without experiencing adverse effects.
Delayed hypersensitivity or photodermatitis has been docu-
mented for St John's wort following the ingestion of a herbal tea made from the leaves.(176)
Psychiatric effects Cases of mania(177, 178) and hypomania(179, 180) have been reported in individuals taking St John's wort preparations. Two cases of mania were reported in patients with
bipolar depression who began self-treatment with standardised St John's wort extract (900 mg) daily(178) and one in a patient
experiencing a moderate depressive episode who was taking both sertraline and St John's wort (dosage not known).(177) A case of
hypomania was reported in a woman with panic disorder and unipolar major depression who had discontinued sertraline treatment one week before starting St John's wort tincture.(179) Two cases of hypomania were reported in individuals with no history of bipolar disorder.(180) A man who had received electroconvulsive therapy and who had previously taken various antidepressant drugs, including venlafaxine, fluvoxamine, moclobemide and nortriptyline, experienced a hypomanic episode six weeks after starting St John's wort (dosage not stated). A man with symptoms of post-traumatic stress disorder was diagnosed
with an acute manic episode after three months of self-treatment with St John's wort (dosage not stated).(180)
The cases described above, along with five other cases of mania, one of hypomania, two of schizophrenia, two of delirium and one of acute anxiety, were included in a review of psychotic events associated with administration of St John's wort products. Across the 17 cases, the onset of symptoms ranged from two days to six months after initiation of St John's wort treatment.(181) The dosage of St John's wort taken was stated in only six of these cases; this was within recommended dosages except in one case where an excessively high dose was reported to have been taken (18 g; no further details provided). All these reports stated that the symptoms had resolved after stopping treatment with St John's wort, although in one case the patient improved but initially remained agitated despite cessation of St John's wort.(180) None of the cases involved rechallenge with St John's wort. Typically, these cases have involved self-treatment with St John's wort products, rely on self-report of St John's wort use, have not obtained blood samples for analysis of constituents of H. perforatum and/or their metabolites and have not obtained samples of the product for analysis. Also, in most cases, there were other pharmacological factors and/or underlying illnesses that could have been responsible for or contributed to the observed events.
Other effects A case of subacute toxic neuropathy possibly
related to the use of St John's wort and subsequent exposure to sunlight has been reported.(182) A woman developed stinging pains
in areas exposed to the sun (face and hands) four weeks after starting treatment with St John's wort (500 mg/day, extract and hypericin content not stated); the report did not state whether the woman was using any other products. Her symptoms improved

three weeks after stopping St John's wort and disappeared over the next two months.
There have been reports of sensory nerve hypersensitivity
occurring in individuals who have taken St John's wort preparations (tablets or tinctures).(183)
There is an isolated report of confusion, disorientation and hypertensive crisis (blood pressure 210/140 mmHg) in a 41-year- old man seven days after he began taking a St John's wort product for work-related stress. It was reported that the man had consumed aged cheese and red wine before onset of the delirium, which is suggestive of hypertension associated with the use of a monoamine oxidase inhibitor.(184) However, although previous in vitro experiments have indicated that H. perforatum extracts had monoamine oxidase inhibitory activity, this has not been confirmed in subsequent in vitro studies (see Pharmcological Actions, In vitro and animal studies), and consensus is that St John's wort is not a monoamine oxidase inhibitor.(118) In addition, no analysis of blood samples or of the St John's product appears to have been undertaken, thus alternative explanations for the event cannot be ruled out.
St John's wort extract (BNO-1385, containing 255–285 mg extract equivalent to hypericin 900 mg) 255 to 285 mg three times daily taken for 14 days had no significant effect on heart rate variability, cognitive performance and parameters of autonomic function (vasoconstrictory response of cutaneous blood flow and skin conductance response following a single deep inspiration) according to the results of a randomised, double-blind, placebocontrolled, three-arm, crossover trial involving 12 healthy male volunteers. (Decrease in heart rate variability is an effect observed with chronic administration of tricyclic antidepressant agents,
although the implications of this for morbidity and mortality in patients with depression are not yet understood.)(185, 186) In
contrast, amitriptyline 25 mg daily significantly decreased heart rate variability and significantly influenced the parameters of autonomic function. The effects of another St John's wort herb extract (Hyperiforte, 300 mg extract containing hypericin 990 mg,
pseudohypericin |
526 mg |
and |
hyperforin 9–15 mg) |
900 mg or |
1800 mg as a |
single |
dose |
on cognitive and |
psychomotor |
performance were compared with those of amitriptyline 25 mg in a randomised, placebo-controlled, crossover study involving 13 healthy volunteers. Recipients of H. perforatum extract, compared with placebo, experienced a dose-dependent impairment in one of the eight tests (digit symbol substitution test), whereas amitripty-
line recipients showed statistically significant impairments in performance in seven of the eight tests.(187)
In a case–control study, 37 patients with raised serum thyroidstimulating hormone (TSH) concentrations (7–20 mU/mL) matched for age and sex to 37 individuals with normal TSH concentrations (1–3 mU/mL) selected from the same set of patient records, were interviewed regarding their exposure to St John's wort products and other prescription and non-prescription medicines. Four participants with raised TSH concentrations and two with normal TSH concentrations reported that they had taken St John's wort products within six months of the TSH test (odds ratio, 95% CI for raised TSH concentrations associated
with self-reported exposure to St John's wort during the specified time period: 2.12, 0.36 to 12.36; p > 0.05).(188) The small sample
size may mean that the study lacked the statistical power to detect an association between St John's wort exposure and raised TSH concentrations. Other methodological issues include the possibility of confounding by indication, since individuals with hypothyroidism may experience symptoms of depression and,
St John’s Wort |
561 |
|
therefore, be more likely to take St John's wort.(188) Further |
|
|
research to assess the possibility of effects of H. perforatum on |
|
|
thyroid function may be warranted. |
|
|
There are two isolated reports of sexual dysfunction, including |
|
|
decreased libido and erectile dysfunction, associated with use of St |
|
|
John's wort products. In one man, previous symptoms of sexual |
|
|
dysfunction, possibly related to use of a conventional antidepres- |
|
|
sant agent which had resolved on stopping treatment, reappeared |
|
|
one week after initiation of treatment with St John's wort.(189) |
|
|
Another report describes a man who experienced decreased libido |
|
|
whilst taking St John's wort for nine months.(190) |
|
|
Preclinical data |
|
|
The consumption of large quantities of St John's wort by grazing |
|
|
animals has been associated with the development of photo- |
|
|
sensitivity.(191, G22, G51) Mice given 0.2–0.5 mg of the |
herb were |
|
found to develop severe photodynamic effects.(G22) Studies using |
|
|
cell cultures of human keratinocytes incubated with hypericin or |
|
|
St John's wort extract and exposed to UV-A resulted in a |
|
|
reduction in the LC50 (lethal concentration) with hypericin, but |
|
|
only a mild reduction with hypericum.(192) From these findings it |
|
|
has been estimated that at least 30 times the therapeutic dose |
|
|
would be necessary to produce phototoxic effects in humans.(192) |
|
|
It has been shown in in vitro experiments using a human |
|
|
keratinocyte cell line and quercitrin-free and quercitrin-containing |
|
|
extracts of H. perforatum that quercitrin reduces the photo- |
|
|
toxicity of H. perforatum extracts.(193) |
|
|
Experimental evidence has suggested that a solution of |
|
|
hypericin can react with visible and UV light to produce free |
|
|
radical species and that this may lead to damage of proteins in the |
|
|
lens of the eye.(194) Up to October 2005, there were no |
|
|
spontaneous reports of cataract formation in individuals who |
|
|
have taken St John's wort.(195) |
|
|
A hydromethanolic extract of St John's wort flowering tops |
|
|
(containing 0.3% hypericin) at concentrations of 1–300 mg/mL |
|
|
reduced experimentally induced contractions in rat vas deferens |
|
|
smooth muscle in a concentration-dependent manner.(196) The St |
|
|
John's wort extract and hyperforin also produced a concentration- |
|
|
dependent inhibition of phenylephrine-induced contractions in |
|
|
human vas deferens in vitro; mean (standard error of mean) IC50 |
|
|
values for the extract and hyperforin were 13.9 (2.0) and 0.45 |
|
|
(0.04) mg/mL, respectively. |
|
|
A number of experimental studies has investigated the |
S |
|
genotoxic potential and mutagenic activity of St John's wort |
||
extracts in vitro and in vivo. In vivo studies and most in vitro |
||
studies provided negative results, indicating a lack of mutagenic potential with defined St John's wort extracts.(G52) Mutagenic activity observed in an in vitro Ames test was attributed to the presence of quercetin, although other studies have found no mutagenic potential with a St John's wort extract and it has been
stated that there is no valid evidence for the carcinogenicity of quercetin in humans.(G21, G52)
Dietary administration of St John's wort to rats was found to have no effect on various hepatic drug-metabolising enzymes (e.g. aminopyrine, N-demethylase, glutathione S-transferase and epoxide hydrolase) or on copper concentrations in the liver (see Contra-indications, Warnings, Drug interactions). No major effects were observed on hepatic iron or zinc concentrations and no significant tissue lesions were found in four rats fed St John's wort in their daily diet for 119 days (10% for first 12 days and 5% thereafter because of unpalatability).(197)

562 St John’s Wort
Cytotoxic constituents related to hyperforin have been isolated from two related Hypericum species (see Pharmacological Actions, In vitro and animal studies).
Contra-indications, Warnings
Individuals with sensitivity towards St John's wort may experience allergic reactions. The use of St John's wort is not advised in known cases of photosensitivity and, in view of the potential of hypericin as a photosensitising agent, therapeutic UV treatment should be avoided whilst using St John's wort.(G1)
It has previously been suggested that excessive doses of St John's wort may potentiate monoamine oxidase inhibitor therapy.(198) However, as monoamine oxidase inhibitory activity has not been reported in vivo with St John's wort, this warning is no longer considered necessary. In addition, avoidance of foodstuffs, such as those containing tyramine (e.g. cheese, wine, meat and yeast extracts) and medicines containing sympathomimetic agents (e.g. cough/cold remedies), which interact with MAOIs, is not considered necessary.
Drug interactions There are important pharmacokinetic interactions and potential for important pharmacodynamic
interactions between St John's wort preparations and certain other medicines.(118, 199, 200)
Evidence for pharmacokinetic interactions between St John's wort preparations and certain other medicines, leading to a loss of
or reduction in the therapeutic effect of those medicines, includes spontaneous reports(199) and published case reports.(200, G79) Drugs
that may be affected include certain anticonvulsants, ciclosporin, digoxin, indinavir (and other HIV protease inhibitors, and HIV non-nucleoside reverse transcriptase inhibitors), oral contraceptives, theophylline and warfarin. A report involving four cases describes reduced plasma methadone trough concentrations in addicts who received St John's wort extract 900 mg daily before methadone maintenance treatment.(201)
There have also been reports of increased serotonergic effects (i. e. pharmacodynamic interactions) in patients taking St John's wort products concurrently with selective serotonin reuptake inhibitors (e.g. sertraline, paroxetine).(202, 203) There are isolated reports of hypotension during general anaesthesia(204) and delayed emergence following general anaesthesia.(205) In both cases, patients had been taking St John's wort before receiving several anaesthetic agents, including fentanyl and propofol. It has been
Ssuggested that treatment with St John's wort preparations should be stopped at least five days before undergoing elective surgery.(206)
In the year 2000, the UK Committee on Safety of Medicines (CSM) issued advice to pharmacists, doctors, other healthcare
professionals and patients on the use of St John's wort products with certain other medicines.(207, 208) The CSM's advice for healthcare professionals regarding patients taking St John's wort and other medicines concurrently can be summarised as follows.
Warfarin, ciclosporin, digoxin, theophylline and anticonvulsants (carbamazepine, phenobarbital and phenytoin)
There is a risk of reduced therapeutic effect, e.g. risk of transplant rejection, seizures and loss of asthma control. Advice is to check plasma drug concentrations (with warfarin, the patient's International Normalised Ratio should be checked) and to stop St John's wort therapy. In addition, dose adjustment may be necessary.
HIV protease inhibitors (indinavir, nelfinavir, ritonavir and saquinavir) and HIV non-nucleoside reverse transcriptase inhibitors (efavirenz and nevirapine) There is a risk of reduced blood concentrations with possible loss of HIV suppression. Advice is to measure HIV RNA viral load and to stop St John's wort.
Oral contraceptives There is a risk of reduced blood concentrations, breakthrough bleeding and unintended pregnancy. Advice is to stop St John's wort.
Triptans (sumatriptan, naratriptan, rizatriptan and zolmitriptan) and selective serotonin reuptake inhibitors (citalopram, fluoxetine, fluvoxamine, paroxetine and sertraline)
There is a risk of increased serotonergic effects with the possibility of an increased risk of adverse reactions. Advice is to stop St John's wort.
Patients already taking any of the above drugs should be advised not to start taking St John's wort and users of other medicines should be advised to seek professional advice before using St John's wort. Topical medicines and non-psychotropic medicines that are excreted renally are not likely to interact with St John's wort. In addition, topical or homeopathic preparations of St John's wort are not likely to interact with prescribed medicines.
There is further evidence from prospective pharmacokinetic studies involving patients who have received St John's wort preparations and other medicines concurrently that there are interactions which result in altered pharmacokinetics, includ-
ing reduced plasma concentrations, of amitriptyline,(209) ciclosporin,(210, 211) tacrolimus,(212) and irinotecan.(213) These
studies have typically been open-label studies (although two involved a randomised, crossover design) involving small numbers of patients undergoing treatment with the respective conventional medicines and who were also treated with single and/or multiple doses (600 to 900 mg extract daily for around two weeks) of St John's wort preparations. In one randomised, crossover study, 10 patients who had undergone renal transplants were treated concurrently with ciclosporin and St John's wort extract with a low or high hyperforin content. Patients experienced altered ciclosporin pharmacokinetics and required ciclosporin dose increases only whilst taking the extract with the high content of hyperforin.(211)
Studies with different designs (and varying methodological quality)(214) and involving healthy volunteers have provided supporting evidence of pharmacokinetic interactions between St John's wort preparations and digoxin,(215) imatinib,(216, 217) oral contraceptives,(218, 219) phenprocoumon,(220) quazepam,(221) simvastatin,(222) tacrolimus,(223) verapamil(224) and warfarin.(225) These and other studies provide evidence indicating that St John's wort preparations induce the cytochrome P450 (CYP)
drug-metabolising enzyme CYP3A4,(220, 226–229) as |
well as |
affecting P-glycoprotein (a transport protein).(200, 230) |
As with |
other medicines, whether or not clinically important drug interactions occur with St John's wort preparations depends on several factors, including the dosage regimen, route of administration and pharmaceutical quality of St John's wort preparations and co-administered medicines. As CYP3A4 is involved in the metabolism of at least half of all medicinal agents, and as P- glycoprotein is involved in the transport of many drugs, it is possible that St John's wort preparations interact with other medicines in addition to those listed above.(231)

There is also evidence from studies involving healthy
volunteers, albeit less extensive, that St John's wort preparations induce CYP2C19.(232) Effects on certain other CYP drug
metabolising enzymes are less clear: one study reported no effect on CYP2C9 activity,(229) whereas another study found that a St John's wort extract did induce CYP2C9 as determined by effects on the pharmacokinetics of S-warfarin;(225) conflict-
ing results have also been reported for CYP1A2 with respect to induction(225) or a lack of induction(229) by St John's wort
preparations.
In contrast, similar studies have reported a lack of pharmacokinetic interaction between St John's wort prepara-
tions and carbamazepine,(233) pravastatin,(222) and theophylline,(234) and several others have reported a lack of significant
effects on CYP isoenzymes,(235–237) although the numbers of volunteers may have been too small and the duration of St John's wort administration too short to exclude definitively an inductive effect.(236, 237) There is evidence from several studies involving healthy volunteers that St John's wort preparations
do not influence CYP2D6 activity to an extent likely to be clinically relevant.(200, 228, 229, 238)
Randomised, placebo-controlled studies involving healthy volunteers who received an ethanol extract of St John's wort with a low hyperforin content (Esbericum capsules containing 60 mg extract, drug to extract ratio 3.5–6.0 : 1, equivalent to 0.25 mg total hypericins and 0.88 mg hyperforin) 240 mg extract daily found no statistically significant effects on the pharmacokinetics of drugs used as substrates for CYP3A4, CYP1A2 and CYP2C9. It is not clear whether the lack of effect
is due to the preparation having a low hyperforin content, or to the low dose of extract used in the studies.(239) The suggestion
that the occurrence of CYP enzyme induction, or the extent of induction, may vary depending on the particular St John's wort preparation is a valid one, since the profile of constituents, including those influencing CYP enzyme activity, varies qualitatively and quantitatively between products (see Quality of plant material and commercial products).
A further randomised, double-blind, placebo-controlled study involving healthy volunteers (n = 33) explored the effects of the CYP enzyme inhibitor cimetidine and the CYP enzyme inducer carbamazepine on the pharmacokinetics of certain constituents of St John's wort extracts. Participants received St John's wort extract (LI-160) 300 mg three times daily for 11 days, followed by a further seven days' administration together with cimetidine 1000 mg daily in divided doses, carbamazepine 400 mg each night, or placebo. No statistically significant differences in the plasma concentration versus time curves from hours 0 to 24 (AUC0–24) were observed between groups; statistically significant intragroup differences in the AUC0–24 were observed for hypericin during cimetidine administration and for pseudohypericin during carbamazepine administration,
although these effects are unlikely to be of clinical relevance.(240)
A number of in vitro and animal studies have also explored the effects of St John's wort preparations on CYP drug metabolising enzymes. In vitro studies have reported induction
of the CYP enzymes CYP3A4 and CYP1A2 by St John's wort extracts,(200, 231) and of CYP2C9 by hyperforin.(241, 242) Results
from in vivo (mice) experiments indicate that hyperforin is important for induction of CYP3A in the liver.(243) In vitro
experiments have shown that hyperforin induction of CYP2C9
is mediated by the pregnane X nuclear receptor.(242) Induction of P-glycoprotein by St John's wort extract(244) and hyper-
St John’s Wort |
563 |
forin(245) has been reported following in vitro experiments using LS180 intestinal carcinoma cells, whereas there are
conflicting results on the induction of P-glycoprotein by hypericin.(244, 245)
A number of in vitro experiments have described inhibition of the CYP enzymes CYP3A4 and CYP2C19 by St John's wort extracts,(246, 247) and inhibition of P-glycoprotein by hypericin and hyperforin.(248) A series of in vitro experiments explored the effects of hypericin on human DNA topoisomerase II activity. Hypericin appeared to interact with DNA in a manner which precluded topoisomerase II DNA binding activity and antagonised the formation of topoisomerase II-covalent cleavage complexes mediated by the topoisomerase II poisons etoposide and amsacrine. The effects of hypericin and St John's wort extracts on topoisomerase II cancer chemotherapy require further investigation.(249)
Certain groups of users may have little knowledge of safety aspects, such as drug interactions, related to use of St John's
wort products, and may be particularly vulnerable to certain adverse effects.(250–253)
Pregnancy and lactation Information on the use of H. perforatum preparations during pregnancy and breastfeeding is summarised below. In view of the lack of toxicity data, St John's wort preparations should not be used during pregnancy and lactation.
Clinical data In a prospective, cohort study, 33 breastfeeding women who had made enquiries about the safety of St John's wort during breastfeeding to a teratogen/toxicology advice service (and who took St John's wort products) were compared with 33 ageand parity-matched controls and 101 diseasematched controls (who had enquired about the safety of St John's wort products but who did not take any). Three of the women in the first group had initiated St John's wort treatment during pregnancy (stage not stated), and the mean (standard
deviation) of infant exposure to St John's wort through breastfeeding was 2.1 (3.5) months.(254) There were no
statistically significant differences in maternal or infant demographics, and in women's reasons for enquiring about use of St John's wort, although significantly more women who took St John's wort were using conventional antidepressants, compared with women who inquired about St John's wort but who did not use it (42.4% versus 17.8%; p < 0.01). No maternal adverse events were reported in any of the women and
there were no statistically significant differences in the S proportions of women reporting decreased milk volume
(12.1%, 6.9% and 6.1% for St John's wort consumers,
disease-matched controls, and age-/parity-matched controls, respectively; p = 0.58).(254) Five infants born to St John's wort
consumers experienced colic, drowsiness or lethargy, compared with one report in each of the other two groups, although two of the former five infants were also exposed to conventional antidepressant agents during breastfeeding.
The study described above provides only limited information on the safety of St John's wort products during breastfeeding (and, to a even lesser extent, pregnancy) as it involved only small numbers of women so had the statistical power to detect only very common, acute adverse events. In addition, a report of the study did not provide any details of the St John's wort products consumed by the participants. It is likely that several, perhaps many, different St John's wort products were involved, all of which are likely to differ in their pharmaceutical quality and, therefore, in their potential effects on the infant.

564 St John’s Wort
There is a report of a 38-year-old woman who started taking
St John's wort (900 mg/day) at her 24th week of pregnancy, taking the last dose 24 hours before delivery.(255) The pregnancy
was unremarkable except for late onset of thrombocytopenia. Another report described a 43-year-old woman who discontinued fluoxetine and methylphenidate upon becoming preg-
nant and started taking St John's wort (900 mg/day). The report does not state the outcome of the pregnancy,(255)
although it is assumed that had adverse events occurred, they would have been stated.
Another case report describes a 33-year-old woman who presented with post-natal depression at a German psychiatric service and who had been taking an extract of H. perforatum (Jarsin) 300 mg three times daily from five months postpartum. Analysis of breast milk samples indicated that
hyperforin is excreted into breast milk at concentrations below the lower limit of quantification (0.50 ng/mL).(256) No adverse
effects were observed in the infant or the mother, however, further investigation of the pharmacokinetics of constituents of H. perforatum extract in breast milk and in mothers' and infants' plasma is required.
Preclinical data In a randomised experiment, 40 adult female mice were given food bars containing 'hypericum herb' (no further details of plant material provided) at a dose of 180 mg/kg body weight daily, or placebo, for two weeks before conception and throughout the gestation period. At the end of the study, there were no statistically significant differences between the 'hypericum' and placebo groups in duration of gestation, number of live pups per litter, body length and head circumference measurements, dam–pup interactions, physical
maturation milestones and reproductive capabilities of offspring.(257) The only exceptions to this were that male offspring
in the hypericum-exposed group weighed significantly less than those in the placebo group at birth (p < 0.02), but this difference was not statistically significant by day three after birth, and male offspring in the hypericum-exposed group experienced a temporary delay in appearance of the upper incisors. Since multiple statistical tests were carried out, this number of statistically significant results at a level of p < 0.05 is likely. In similar experiments, 18 adult female rats were given a methanol extract of St John's wort (containing 0.3% total hypericins) 100 or 1000 mg/kg, or placebo, by gavage for two weeks before mating, throughout the gestation period and/or for three weeks
Sduring breastfeeding. Histological alterations in the livers and kidneys of rat pups exposed to St John's wort extract throughout gestation and/or during breastfeeding; lesions were more severe in the offspring of rats treated with the
higher dose and in offspring exposed to St John's wort extract during both the gestation period and breastfeeding.(258) Slight in vitro uterotonic activity has been reported for St John's wort (see Pharmacological Actions, In vitro and animal studies).
Preparations
Proprietary single-ingredient preparations
Argentina: Amenicil; Hipax; Hiperinat. Australia: Bioglan Stress-Relax; Hyperiforte. Austria: Esbericum; Helarium; Hyperiforce; Jarsin; Johanicum; Johni; Kira; Lunare; Perikan; Psychotonin; Remotiv; Solaguttae. Belgium: Hyperiplant; Milperinol; Perika. Brazil: Adprex; Emotival; Equilibra;
Fiotan; Hiperex; Hipericin; Hiperico; Hiperifarma; Hiperil; Hipersac; Hyperico; Hyperigreen; Iperisan; Jarsin; Motiven; Prazen; Triativ. Canada: Kira; Movana. Chile: Anxium; Edual. Czech Republic: Deprim; Lubovnik; Nat Trezalky; Trezalka v Nalevovych Sacchich; Trezalkova Nat; Trezalkovy Caj; Turineurin. France: Dermum; Procalmil. Germany: Aristo; Aristoforat; Cesradyston; Digitalysat Scilla-Digitaloid; dystolux; Esbericum; Felis; Helarium; Hewepsychon uno; Hyperforat; Hypericaps; Hyperimerck; Hyperpur; Jarsin; Jo-Sabona; Kira; Laif; Libertin; Lomahypericum; Nervei; Neuroplant; Neurosporal; Neurovegetalin; Psychotonin; Sedovegan; Syxal; Texx; Tonizin; Turineurin. Hungary: Helarium; Remotiv. Italy: Nervaxon; Proserem; Quiens; Remotive. Mexico: Hiperikan; Procalm; Remotiv. Russia: Deprim (Деприм); Helarium (Гелариум); Negrustin (Негрустин); Novo-Passit (Ново-Пас- сит). Spain: Animic; Arkocapsulas Hiperico; Hiperico; Hyneurin; Quetzal; Vitalium. Switzerland: Hyperiforce; HyperiMed; Hyperiplant; Hyperval; Jarsin; Lucilium; ReBalance; Remotiv; Solevita; Yakona. UK: Kira. Venezuela: Kira.
Proprietary multi-ingredient preparations
Australia: Bioglan 3B Beer Belly Buster; Cimicifuga Compound; Feminine Herbal Complex; Infant Tonic; Irontona; Joint & Muscle Relief Cream; Nappy Rash Relief Cream; Nevaton; Skin Healing Cream; Vitatona. Austria: Eryval; Magentee St Severin; Nerventee St Severin; Remifemin plus; Species nervinae; Wechseltee St Severin. Canada: Bronco Asmol; Sirop Cocillana Codeine. Czech Republic: Alvisan Neo; Cajova Smes pri Redukcni Diete; Cicaderma; Eugastrin; Fytokliman Planta; Naturident; Novo-Passit; Species Nervinae Planta; Stomaran; Zaludecni Cajova Smes. Germany: anabolloges; Gastritol; Gutnacht; Hyperesa; Me-Sabona plus; Miroton; Neurapas; Remifemin plus; Sedariston Konzentrat; Sedariston plus. Hong Kong: Coci-Fedra; Cocillana Christo; Cocillana Compound; Cocillana Compound; Cocillana Compound; Mefedra-N; Mist Expect Stim. Italy: Hiperogyn; Mithen. Mexico: Nordimenty. Portugal: Cicaderma. Russia: Prostanorm (Простанорм). South Africa: Cocillana Co; Contra-Coff; Linctus Tussi Infans. Spain: Natusor Gastrolen; Natusor Somnisedan. Switzerland: Gel a la consoude; Huile de millepertuis A. Vogel (huile de St. Jean); Hyperiforce comp; The a l'avoine sauvage de Vollmer. UK: Allens Chesty Cough; Balm of Gilead; Buttercup Syrup; Chest Mixture; Covonia Mentholated; Galloway's Cough Syrup; Honey & Molasses; Modern Herbals Cough Mixture; Potters Children's Cough Pastilles; Potters Gees Linctus; Sanderson's Throat Specific; Sanderson's Throat Specific; St Johnswort Compound. Venezuela: Biomicovo.
References
1Bombardelli E, Morazzoni P. Hypericum perforatum. Fitoterapia
1995; 66: 43–68.
2Hölzl J, Petersen M. Chemical constituents of Hypericum spp. In Ernst E (ed), Hypericum. The genus Hypericum. London, New York:
Taylor and Francis, 2003.
3Vanhaelen M, Vanhaelen-Fastre R. Quantitative determination of biologically active constituents in medicinal plant crude extracts by thin-layer chromatography-densitometry. J Chromatogr 1983; 281:
263–271.
4Berghöfer R, Hölzl J. Biflavonoids in Hypericum perforatum; part 1. Isolation of 13,II8-biapigenin. Planta Med 1987; 53: 216–217.

5Berghöfer R, Hölzl J. Isolation of I30,II8-biapigenin (amentoflavone) from Hypericum perforatum. Planta Med 1989; 55: 91.
6Ollivier B et al. Separation et identification des acides phenols par chromatographie liquide haute performance et spectroscopie ultraviolette. Application à la pariétaire (Parietaria officinalis L.) et au
millepertuis (Hypericum perforatum L.). J Pharm Belg 1985; 40: 173– 177.
7Hoelzl J, Ostrowski E. St John's wort (Hypericum perforatum L.). HPLC analysis of the main components and their variability in a
population. Dtsch Apoth Ztg 1987; 127: 1227–1230.
8 Dorossiev I. Determination of flavonoids in Hypericum perforatum. Pharmazie 1985; 40: 585–586.
9 Brondz I et al. The relative stereochemistry of hyperforin – an antibiotic from Hypericum perforatum L. Tetrahedron Lett 1982; 23: 1299–1300.
10Ayuga C, Rebuelta M. A comparative study of phenolic acids of
Hypericum caprifolium Boiss and Hypericum perforatum L. An Real Acad Farm 1986; 52: 723–728.
11Brondz I et al. n-Alkanes of Hypericum perforatum: a revision. Phytochemistry 1983; 22: 295–296.
12Mathis C, Ourisson G. Étude chimio-taxonomique du genre Hypericum – II. Identification de constituants de diverses huiles essentielles d'Hypericum. Phytochemistry 1964; 3: 115–131.
13Mathis C, Ourisson G. Étude chimio-taxonomique du genre Hypericum – IV. Repartition des sesquiterpenes, des alcools monoterpeniques et des aldehydes satures dans les huiles essentielles d'Hypericum. Phytochemistry 1964; 3: 377–378.
14Mathis C, Ourisson G. Étude chimio-taxonomique du genre Hypericum – V. Identification de quelques constituants non volatils d'Hypericum perforatum L. Phytochemistry 1964; 3: 379.
15ConsumerLab. Product Review: St John's wort. (accessed 28 August 2003).
16De Los Reyes GC, Koda RT. Determining hyperforin and hypericin content in eight brands of St John's wort. Am J Health-Syst Pharm 2002; 59: 545–547.
17Draves AH, Walker SE. Analysis of the hypericin and pseudohypericin content of commercially available St John's wort preparations. Can J Clin Pharmacol 2003; 10(3): 114–118.
18Orth HCJ et al. Isolation, purity analysis and stability of hyperforin as a standard material from Hypericum perforatum L. J Pharm Pharmacol 1999; 51: 193–200.
19Vaja V et al. Further degradation product of hyperforin from
Hypericum perforatum (St. John's wort). Fitoterapia 2003; 74: 439– 444.
20Verotta L et al. Furohyperforin, a prenylated phloroglucinol from St John's wort (Hypericum perforatum). J Nat Prod 1999; 62: 770–772.
21Verotta L et al. Hyperforin analogues from St John's wort (Hypericum perforatum). J Nat Prod 2000; 63: 412–415.
22Tolonen A et al. Fast high-performance liquid chromatographic analysis of naphthodianthrones and phloroglucinols from Hypericum perforatum extracts. Phytochemical Anal 2003; 14: 306–309.
23Westerhoff K et al. Biorelevant dissolution testing of St John's wort products. J Pharm Pharmacol 2002; 54: 1615–1621.
24Linde K et al. St John's wort for depression. The Cochrane Database of Systematic Reviews, 2005, Issue 3. Art. No. :CD000448.pub2.
25Nahrstedt A, Butterweck V. Biologically active and other chemical constituents of the herb of Hypericum perforatum L. Pharmacopsychiatry 1997; 30 (Suppl. Suppl.): 129–134.
26Butterweck V et al. Plasma levels of hypericin in presence of procyanidin B2 and hyperoside: a pharmacokinetic study in rats. Planta Med 2003; 6: 189–192.
27Suzuki O et al. Inhibition of monoamine oxidase by hypericin. Planta Med 1984; 50: 272–274.
28Bladt S, Wagner H. Inhibition of MAO by fractions and constituents of Hypericum extract. J Geriat Psychiatr Neurol 1994; 7: S57–59.
29Demisch L et al. Identification of MAO-type-A inhibitors in
Hypericum perforatum L. (Hyperforat). Pharmacopsychiatry 1989;
22:194.
30Thiede HM, Walper A. Inhibition of MAO and COMT by
Hypericum extracts and hypericin. J Geriat Psychiatr Neurol 1994; 7: S54–56.
|
St John’s Wort |
565 |
31 |
Cott JM. In vitro receptor binding and enzyme inhibition by |
|
|
Hypericum perforatum extract. Pharmacopsychiatry 1997; 30 (Suppl. |
|
|
Suppl.): 108–112. |
|
32 |
Müller WE et al. Effects of hypericum extract (LI 160) in biochemical |
|
|
models of antidepressant activity. Pharmacopsychiatry 1997; 30 |
|
|
(Suppl. Suppl.): 102–107. |
|
33 |
Calapai G et al. Effects of Hypericum perforatum on levels of 5- |
|
|
hydroxytryptamine, noradrenaline and dopamine in the cortex, |
|
|
diencephalon and brainstem of the rat. J Pharm Pharmacol 1999; 51: |
|
|
723–728. |
|
34 |
Winkler C et al. St John's wort (Hypericum perforatum) counteracts |
|
|
cytokine-induced tryptophan catabolism in vitro. Biol Chem 2004; |
|
|
385: 1197–1202. |
|
35 |
Zanoli P. Role of hyperforin in the pharmacological activities of St |
|
|
John's wort. CNS Drug Rev 2004; 10: 203–218. |
|
36 |
Chatterjee SS et al. Hyperforin as a possible antidepressant |
|
|
component of hypericum extracts. Life Sci 1998; 63: 499–510. |
|
37 |
Chatterjee SS et al. Antidepressant activity of Hypericum perforatum |
|
|
and hyperforin: the neglected possibility. Pharmacopsychiatry 1998; 31 |
|
|
(Suppl. Suppl.): 7–15. |
|
38 |
Gobbi M et al. Hypericum perforatum L. extract does not inhibit 5- |
|
|
HT transporter in rat brain cortex. Naunyn-Schmiedebergs Arch |
|
|
Pharmacol 1999; 360: 262–269. |
|
39 |
Jensen AG et al. Adhyperforin as a contributor to the effect of |
|
|
Hypericum perforatum L. in biochemical models of antidepressant |
|
|
activity. Life Sci 2001; 68: 1593–1605. |
|
40 |
Butterweck V et al. In vitro receptor screening of pure constituents of |
|
|
St John's wort reveals novel interactions with a number of GPCRs. |
|
|
Psychopharmacology 2002; 162: 193–202. |
|
41 |
Fiebich BL et al. Inhibition of substance P-induced cytokine synthesis |
|
|
by St John's wort extracts. Pharmacopsychiatry 2001; 34 (Suppl. |
|
|
Suppl. 1): S26–S28. |
|
42 |
Gobbi M et al. In vitro effects of the dicyclohexylammonium salt of |
|
|
hyperforin on interleukin-6 release in different experimental models. |
|
|
Planta Med 2004; 70: 680–682. |
|
43 |
Butterweck V et al. Step by step removal of hyperforin and hypericin: |
|
|
activity profile of different Hypericum preparations in behavioral |
|
|
models. Life Sci 2003; 73: 627–639. |
|
44 |
Butterweck V. Mechanism of action of St John's wort in depression: |
|
|
what is known?. CNS Drugs 2003; 17: 539–562. |
|
45 |
Singer A et al. Hyperforin, a major antidepressant constituent of St. |
|
|
John's wort, inhibits serotonin uptake by elevating free intracellular |
|
|
Naþ. J Pharmacol Exp Ther 1999; 290: 1363–1368. |
|
46 |
Singer A et al. Hyperforin alters free intracellular Hþ and Naþ |
|
|
concentration in human platelets (abstract). Paper presented at the |
|
|
Biocenter Symposium on Drug Therapy. Pharmacology of St. John's |
|
|
Wort (Hypericum perforatum L.) and its Constituents., February |
|
|
2000, Frankfurt, Germany. |
|
47 |
Treiber K et al. Hyperforin activates nonselective cation channels |
|
|
(NSCCs). Br J Pharmacol 2005; 145: 75–83. |
|
48 |
Uebelhack R, Franke L. In vitro effects of hypericum extract and |
|
hyperforin on 5HT uptake and efflux in human blood platelets |
S |
|
(abstract). Paper presented at the Biocenter Symposium on Drug |
||
|
||
Therapy. Pharmacology of St. John's Wort (Hypericum perforatum L.) |
|
|
and its Constituents, February 2000, Frankfurt, Germany. |
|
49Okpanyi SN, Weischer ML. Animal experiments on the psychotropic action of a Hypericum extract. Arzneimittelforschung 1987; 37: 10–13.
50Gambarana C et al. Efficacy of an Hypericum perforatum (St John's wort) extract in preventing and reverting a condition of escape deficit in rats. Neuropsychopharmacology 1999; 21: 247–257.
51De Vry J et al. Comparison of hypericum extracts with imipramine and fluoxetine in animal models of depression and alcoholism. Eur Neuropsychopharmacol 1999; 9: 461–468.
52Butterweck V et al. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med 2000; 66: 3–6.
53Martarelli D et al. Hypericum perforatum methanolic extract inhibits growth of human prostatic carcinoma cell line orthotopically implanted in nude mice. Cancer Lett 2004; 210: 27–33.
54Hostanska K et al. Aqueous ethanolic extract of St John's wort (Hypericum perforatum L.) induces growth inhibition and apoptosis in human malignant cells in vitro. Pharmazie 2002; 57: 323–331.

566 St John’s Wort
55 Hostanska K et al. Comparison of the growth-inhibitory effect of Hypericum perforatum L. extracts, differing in the concentration of phloroglucinols and flavonoids, on leukaemia cells. J Pharm Pharmacol 2003; 55: 973–980.
56 Roscetti G et al. Cytotoxic activity of Hypericum perforatum L. on K562 erythroleukemic cells: differential effects between methanolic extract and hypericin. Phytotherapy Res 2004; 18: 66–72.
57 Donà M et al. Hyperforin inhibits cancer invasion and metastasis. Cancer Res 2004; 64: 6225–6232.
58 Schempp CM et al. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St John's wort that acts by induction of apoptosis. Oncogene 2002; 21: 1242–1250.
59 Hostanska K et al. Hyperforin a constituent of St John's wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur J Pharm Biopharmaceut 2003; 56: 121–132.
60 Colasanti A et al. Hypericin photosensitization of tumor and metastatic cell lines of human prostate. J Photochem Photobiol B Biology 2000; 54: 103–107.
61 Liu CD et al. Hypericin and photodynamic therapy decreases human pancreatic cancer in vitro and in vivo. J Surg Res 2000; 93: 137–143.
62 Delaey E et al. Photocytotoxicity of hypericin in normoxic and hypoxic conditions. J Photochem Photobiol B Biology 2000; 56: 19–24.
63 Höpfner M et al. Hypericin activated by an incoherent light source has photodynamic effects on esophageal cancer cells. Int J Colorectal Dis 2003; 18: 239–247.
64 Kamuhabwa AR et al. Photodynamic activity of hypericin in human
urinary bladder carcinoma cells. Anticancer Res 2000; 20: 2579–2584. 65 Čarvarga I et al. Photodynamic therapy of murine fibrosarcoma with
topical and systemic administration of hypericin. Phytomedicine 2001; 8: 325–330.
66 Solár P et al. Effect of azetazolamide on hypericin photocytotoxicity. Planta Med 2002; 68: 658–660.
67 Miroššay A et al. The effect of quercetin on light-induced cytotoxicity of hypericin. Physiol Res 2001; 50: 635–637.
68 Schempp CM et al. Phototoxic and apoptosis-inducing capacity of pseudohypericin. Planta Med 2002; 68: 171–173.
69 Schempp CM et al. Hypericin photo-induced apoptosis involves the tumor necrosis factor apoptosis-inducing ligand (TRAIL) and activation of caspase-8. FEBS Lett 2001; 493: 26–30.
70 Ali SM et al. Hypericin and hypocrellin induced apoptosis in human mucosal carcinoma cells. J Photochem Photobiol B Biology 2001; 65: 59–73.
71 Pajonk F et al. Hypericin – an inhibitor of proteasome function.
Cancer Chemother Pharmacol 2005; 55: 439–446.
72 Kamuhabwa AR et al. Cellular photodestruction induced by hypericin in AY-27 rat bladder carcinoma cells. Photochem Photobiol 2001; 74: 126–132.
73 Paba V et al. Photo-activation of hypericin with low doses of light promotes apparent photo-resistance in human histiocytic lymphoma
S |
U937 cells. J Photochem Photobiol B Biology 2001; 60: 87–96. |
|
74Varriale L et al. Molecular aspects of photodynamic therapy: low energy pre-sensitization of hypericin-loaded human endometrial carcinoma cells enhances photo-tolerance, alters gene expression and affects the cell cycle. FEBS Lett 2002; 512: 287–290.
75Schwarz D et al. St John's wort extracts and some of their constituents potently inhibit ultimate carcinogen formation from benzo(a)pyrene- 7,8-didhyrodiol by human CYP1A1. Cancer Res 2003; 63: 8062–8068.
76Decosterd LA et al. Isolation of new cytotoxic constituents from
Hypericum revolutum and Hypericum calycinum by liquid–liquid chromatography. Planta Med 1988; 54: 560.
77Avato P et al. Extracts from St John's wort and their antimicrobial activity. Phytotherapy Res 2004; 18: 230–232.
78Zakharova NS et al. Action of plant extracts on the natural immunity indices of animals. Zh Mikrobiol Epidemiol Immunobiol 1986; 4: 71– 75.
79Schempp C et al. Antibacterial activity of hyperforin from St John's wort, against multiresistant Staphylococcus aureus and Gram-positive bacteria. Lancet 1999; 353: 2129.
80Voss A, Verweij PE. Antibacterial activity of hyperforin from St John's wort. Lancet 1999; 354: 777.
81Fiebich B et al. Antibacterial activity of hyperforin from St John's wort. Lancet 1999; 354: 777.
82Hübner AT et al. Treatment with Hypericum perforatum L. does not trigger decreased resistance in Staphylococcus aureus against antibiotics and hyperforin. Phytomedicine 2003; 10: 206–208.
83Negrash AK et al. Comparative study of chemotherapeutic and pharmacological properties of antimicrobial preparations from common St John's wort. Fitontsidy Mater Soveshch 1969; 198–200.
84Sakar MK et al. Antimicrobial activities of some Hypericum species growing in Turkey. Fitoterapia 1988; 59: 49–52.
85Dall'Agnol R et al. Antimicrobial activity of some Hypericum species. Phytomedicine 2003; 10: 511–516.
86Gibbons S et al. The genus Hypericum – a valuable resource of antiStaphylococcal leads. Fitoterapia 2002; 73: 300–304.
87Mishenkova EL et al. Antiviral properties of St John's wort and preparations produced from it. Tr S'ezda Mikrobiol Ukr 1975; 222– 223.
88Weber ND et al. The antiviral agent hypericin has in vitro activity against HSV-1 through non-specific association with viral and cellular membranes. Antiviral Chem Chemother 1994; 5: 83–90.
89Wood S et al. Antiviral activity of naturally occurring anthraquinones and anthraquinone derivatives. Planta Med 1990; 56: 651–652.
90Jia YR et al. In vitro study on the effects of hypericin and lysine hydrochloride against herpes virus. Chin J Clin Rehab 2004; 8(5): 966– 967.
91Lavie G et al. Studies of the mechanisms of the antiretroviral agents hypericin and pseudohypericin. Proc Natl Acad Sci USA 1989; 86: 5963–5967.
92Lopez-Bazzocchi I et al. Antiviral activity of the photoactive plant pigment hypericin. Photochem Photobiol 1991; 54: 95–98.
93Meruelo D et al. Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: aromatic polycyclic diones hypericin and pseudohypericin. Proc Natl Acad Sci USA 1988; 85: 5230–5234.
94Hudson JB et al. Antiviral activities of hypericin. Antiviral Res 1991; 15: 101–112.
95Taher MM et al. Mood-enhancing antidepressant St John's wort inhibits the activation of human immunodeficiency virus gene expression by ultraviolet light. Life 2002; 54: 357–364.
96Koch E, Chatterjee SS. Hyperforin stimulates intracellular calcium mobilisation and enhances extracellular acidification in DDT1-MF2 smooth muscle cells (abstract). Paper presented at the Biocenter Symposium on Drug Therapy. Pharmacology of St. John's Wort (Hypericum perforatum L.) and its Constituents. February 2000, Frankfurt, Germany.
97Rezvani AH et al. Attenuation of alcohol intake by extract of Hypericum perforatum (St John's wort) in two different strains of alcohol-preferring rats. Alcohol Alcoholism 1999; 34: 699–705.
98Wright CW et al. Correlation of hyperforin content of Hypericum perforatum (St John's wort) extracts with their effects on alcohol drinking in C57BL/6J mice: a preliminary study. J Psychopharmacol 2003; 17: 403–408.
99Catania MA et al. Hypericum perforatum attenuates nicotine withdrawal signs in mice. Psychopharmacology 2003; 169(2): 186–189.
100Ballenger JC et al. Alcohol and central serotonin metabolism in man. Arch Gen Psych Scand 1979; 57: 224–227. Cited by Rezvani AH et al.
Alcohol Alcoholism 1999; 34: 699–705.
101Hughes JR et al. Antidepressants for smoking cessation. The Cochrane Database of Systematic Reviews, 2004, Issue 4. Art. No.: CD000031.
102Shipochliev T et al. Anti-inflammatory action of a group of plant extracts. Vet Med Nauki 1981; 18: 87–94.
103Bork PS et al. Hypericin as a non-oxidant inhibitor of NF-kB. Planta Med 1999; 65: 297–300.
104Takahashi I et al. Hypericin and pseudohypericin specifically inhibit protein kinase C: possible relation to their antiretroviral activity.
Biochem Biophys Res Commun 1989; 165: 1207–1212.
105Agostinis P et al. Photosensitized inhibition of growth factor regulated protein kinases by hypericin. Biochemical Pharmacol 1995; 49: 1615– 1622.
106De Witte P et al. Inhibition of epidermal growth factor receptor tyrosine kinase activity by hypericin. Biochemical Pharmacol 1993; 46: 1929–1936.

|
|
|
St John’s Wort |
567 |
|
107 |
Panossian AG et al. Immunosuppressive effects of hypericin on |
132 |
Hübner WD, Kirste T. Experience with St John's wort (Hypericum |
|
|
|
stimulated human leucocytes: inhibition of the arachidonic acid |
|
perforatum) in children under 12 years with symptoms of depression |
|
|
|
release, leukotriene B4 and interleukin-1 production and activation of |
|
and psychovegetative disturbances. Phytotherapy Res 2001; 15: 367– |
|
|
|
nitric oxide formation. Phytomedicine 1996; 3: 19–28. |
|
370. |
|
|
108 |
Tahara Y et al. The antidepressant hypericin inhibits progression of |
133 |
Findling RL et al. An open-label pilot study of St John's wort in |
|
|
|
experimental proliferative vitreoretinopathy. Curr Eye Res 1999; 19: |
|
juvenile depression. J Am Acad Child Adolesc Psychiatry 2003; 42: |
|
|
|
323–329. |
|
908–914. |
|
|
109 |
Bezáková L et al. Effect of dianthrones and their precursors from |
134 |
Lenoir S et al. No dose-dependent effect of hypericin in hypericum |
|
|
|
Hypericum perforatum L. on lipoxygenase activity. Pharmazie 1999; |
|
extracts for depression. Phytomedicine 1999; 6: 141–146. |
|
|
|
54: 711. |
135 |
Barnes J et al. A pilot, randomised, open, uncontrolled clinical study |
|
|
110 |
Albert D et al. Hyperforin is a dual inhibitor of cyclooxygenase-1 and |
|
of two dosages of St John's wort (Hypericum perforatum L.) herb |
|
|
|
5-lipoxygenase. Biochemical Pharmacol 2002; 64: 1767–1775. |
|
extract as an aid to motivational/behavioural support in smoking |
|
|
111 |
Hunt EJ et al. Effect of St John's wort on free radical production. Life |
|
cessation. Planta Med 2006; 72(4): 378–382. |
|
|
|
Sci 2001; 69: 181–190. |
136 |
Kasper S. Treatment of seasonal affective disorder (SAD) with |
|
|
112 |
Heilmann J et al. Studies on the antioxidative activity of |
|
hypericum extract. Pharmacopsychiatry 1997; 30 (Suppl. Suppl.): 89– |
|
|
|
phloroglucinol derivatives isolated from Hypericum species. Planta |
|
93. |
|
|
|
Med 2003; 69: 202–206. |
137 |
Wheatley D. Hypericum in seasonal affective disorder (SAD). Curr |
|
|
113 |
Melzer R et al. Proanthocyanidins from Hypericum perforatum: |
|
Med Res Opin 1999; 15: 33–37. |
|
|
|
effects on isolated pig coronary arteries. Planta Med 1988; 54: 572– |
138 |
Hypericin – a plant extract with anti-HIV activity. Scrip 1989; 1415: |
|
|
|
573. |
|
29. |
|
|
114 |
Shiplochliev T. Extracts from a group of medicinal plants enhancing |
139 |
Anon. Hypericin HIV trial in Thailand. Scrip 1995; 2019: 25. |
|
|
|
the uterine tonus. Vet Med Nauki 1981; 18: 94–98. |
140 |
Cooper WC, James J. An observational study of the safety and |
|
|
115 |
Jang M-H et al. Protective effect of Hypericum perforatum Linn (St |
|
efficacy of hypericin in HIV-positive subjects (abstract). Int Conf |
|
|
|
John's wort) against hydrogen peroxide-induced apoptosis on human |
|
AIDS 1990; 6: 369. |
|
|
|
neuroblastoma cells. Neuroscience Lett 2002; 329: 177–180. |
141 |
Steinbeck-Klose A, Wernet P. Successful long-term treatment over 40 |
|
|
116 |
Vasilchenko EA et al. Analgesic action of flavonoids of Rhododendron |
|
months of HIV-patients with intravenous hypericin (abstract). Int |
|
|
|
luteum Sweet, Hypericum perforatum L., Lespedeza bicolor Turcz. |
|
Conf AIDS 1993; 9: 470. |
|
|
|
and L. hedysaroides (Pall.) Kitag. Rastit Resur 1986; 22: 12–21. |
142 |
Gulick RM et al. Phase I studies of hypericin, the active compound in |
|
|
117 |
Grujic-Vasic J et al. The examining of isolated tannins and their |
|
St John's wort, as an antiretroviral agent in HIV-infected adults. Ann |
|
|
|
astringent effect. Planta Med 1986; 52 (Suppl. Suppl.): 67–68. |
|
Intern Med 1999; 130: 510–514. |
|
|
118 |
Barnes J et al. St John's wort (Hypericum perforatum L.): a review of |
143 |
Jacobson JM et al. Pharmacokinetics, safety and antiviral effects of |
|
|
|
its chemistry, pharmacology and clinical properties. J Pharm |
|
hypericin, a derivative of St John's wort plant, in patients with chronic |
|
|
|
Pharmacol 2001; 53: 583–600. |
|
hepatitis C virus infection. Antimicrob Agents Chemother 2001; 45: |
|
|
119 |
Laakman G et al. St John's wort in mild to moderate depression: the |
|
517–524. |
|
|
|
relevance of hyperforin for the clinical efficacy. Pharmacopsychiatry |
144 |
Stevinson C et al. Hypericum for fatigue – a pilot study. |
|
|
|
1998; 31 (Suppl. Suppl.): 54–59. |
|
Phytomedicine 1998; 5: 443–447. |
|
|
120 |
Schroeder C et al. Influence of St John's wort on catecholamine |
145 |
Stevinson C, Ernst E. A pilot study of Hypericum perforatum for the |
|
|
|
turnover and cardiovascular regulation in humans. Clin Pharmacol |
|
treatment of premenstrual syndrome. Br J Obstet Gynaecol 2000; 107: |
|
|
|
Ther 2004; 76: 480–489. |
|
870–876. |
|
|
121 |
Franklin M et al. Neuroendocrine evidence for dopaminergic actions |
146 |
Müller T et al. Treatment of somatoform disorders with St John's |
|
|
|
of hypericum extract (LI 160) in healthy volunteers. Biol Psychiatr |
|
wort: a randomized, double-blind and placebo-controlled trial. |
|
|
|
1999; 46: 581–584. |
|
Psychosomatic Med 2004; 66: 538–547. |
|
|
122 |
Laakman G et al. Effects of hypericum extract on adenohypophysial |
147 |
Kobak KA et al. St John's wort versus placebo in social phobia. |
|
|
|
hormone secretion and catechol amine metabolism (abstract). Paper |
|
Results from a placebo-controlled pilot study. J Clin Psychopharmacol |
|
|
|
presented at the Biocenter Symposium on Drug Therapy. |
|
2005; 25(1): 51–58. |
|
|
|
Pharmacology of St. John's Wort (Hypericum perforatum L.) and its |
148 |
Boblitz N et al. Benefit of a fixed drug combination containing St |
|
|
|
Constituents. February 2000, Frankfurt, Germany. |
|
John's wort and black cohosh for climacteric patients – results of a |
|
|
123 |
Schüle C et al. Endocrinological effects of high-dose Hypericum |
|
randomised clinical trial (abstract). FACT 2000; 5: 85. |
|
|
|
perforatum extract WS 5570 in healthy subjects. Neuropsychobiology |
149 |
Schellenberg R et al. Pharmacodynamic effects of two different |
|
|
|
2004; 49: 58–63. |
|
hypericum extracts in healthy volunteers measured by quantitative |
|
|
124 |
Anon. Johanniskraut VS. SSRI – neue Erkentnnisse zur Wirksamkeit |
|
EEG. Pharmacopsychiatry 1998; 31 (Suppl. Suppl.): 44–53. |
|
S |
|
und Verträglichkeit. Nervenheilkunde 2000; 19: 92–93. |
150 |
Sharpley AL et al. Antidepressant-like effect of Hypericum perforatum |
||
|
|
||||
125 |
Philipp M et al. Hypericum extract versus imipramine or placebo in |
|
(St John's wort) on the sleep polysomnogram. Psychopharmacology |
|
|
|
patients with moderate depression: randomized multicentre study of |
|
1998; 139: 286–287. |
|
|
|
treatment for 8 weeks. BMJ 1999; 319: 1534–1539. |
151 |
Colker CM et al. Effects of Citrus aurantium extract, caffeine, and St |
|
|
126 |
Hypericum Depression Trial Study Group. Effect of Hypericum |
|
John's wort on body fat loss, lipid levels, and mood states in |
|
|
|
perforatum (St John's wort) in major depressive disorder. A |
|
overweight healthy adults. Curr Ther Res 1999; 60: 145–153. |
|
|
|
randomized controlled trial. JAMA 2002; 287: 1807–1814. |
152 |
Friede M et al. Alltagssicherheit eines pflanzlichen Antidepressivums |
|
|
127 |
Vitiello B et al. Hyperforin plasma level as a marker of treatment |
|
aus Johanniskraut. Fortschritte Med 1998; 116: 131–135. |
|
|
|
adherence in the National Institutes of Health Hypericum depression |
153 |
Hottenrot K et al. The influence of vitamin E and extract from |
|
|
|
trial. J Clin Psychopharmacol 2005; 25(3): 243–249. |
|
hypericum on the endurance capacity of competitors. A placebo- |
|
|
128 |
Szegedi A et al. Acute treatment of moderate to severe depression with |
|
controlled double-blind study with long-distance runners and |
|
|
|
hypericum extract WS 5570 (St John's wort): randomised controlled |
|
triathletes. Dtsche Zeitschrift Sportmed 1997; 48: 22–27. |
|
|
|
double blind non-inferiority trial versus paroxetine. BMJ 2005; 330: |
154 |
Schempp CM et al. Topical treatment of atopic dermatitis with St |
|
|
|
503. |
|
John's wort cream – a randomized, placebo controlled, double blind |
|
|
129 |
Linde K et al. St John's wort for depression – an overview and meta- |
|
half-side comparison. Phytomedicine 2003; 10 (Suppl. Suppl. IV): 31– |
|
|
|
analysis of randomised clinical trials. BMJ 1996; 313: 253–258. |
|
37. |
|
|
130 |
Kim HL et al. St John's wort for depression. A meta-analysis of well- |
155 |
Sindrup SH et al. St John's wort has no effect on pain in |
|
|
|
defined clinical trials. J Nerv Ment Dis 1999; 187: 532–539. |
|
polyneuropathy. Pain 2000; 91: 361–365. |
|
|
131 |
Linde K, Knüppel L. Large-scale observational studies of hypericum |
156 |
Ellis KA et al. An investigation into the acute nootropic effects of |
|
|
|
extracts in patients with depressive disorders – a systematic review. |
|
Hypericum perforatum L. (St John's wort) in healthy human |
|
|
|
Phytomedicine 2005; 12: 148–157.15693723 |
|
volunteers. Behav Pharmacol 2001; 12: 173–182. |
|
|

568 St John’s Wort
157 Lavagna SM et al. Efficacy of Hypericum and Calendula oils in the epithelial reconstruction of surgical wounds in childbirth with caesarean section. Il Farmaco 2001; 56: 451–453.
158D'Hallewin MA et al. Fluorescence detection of flat bladder carcinoma in situ after intravesical instillation of hypericin. J Urol 2000; 164: 349–351.
159D'Hallewin MA et al. Hypericin-based fluorescence diagnosis of bladder carcinoma. BJU Int 2002; 89: 760–763.
160Staffeldt B et al. Pharmacokinetics of hypericin and pseudohypericin after oral intake of the Hypericum perforatum extract LI 160 in healthy volunteers. J Geriat Psychiatr Neurol 1994; 7: S47–53.
161Biber A et al. Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers. Pharmacopsychiatry 1998; 31 (Suppl. Suppl.1): 36–43.
162Schulz HU et al. Investigation of the bioavailability of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin following single and multiple oral dosing of a hypericum extract containing tablet. Arzneim Forsch 2005; 55(1): 15–22.
163Agrosi M et al. Oral bioavailability of active principles from herbal products in humans. A study on Hypericum perforatum extracts using the soft gelatin capsule technology. Phytomedicine 2000; 7(6): 455–462.
164Kamuhabwa AR et al. Determination of hypericin in human plasma by high-performance liquid chromatography after intravesical administration in patients with transitional cell carcinoma of the bladder. Eur J Pharmaceut Biopharmaceut 2005; 59: 469–474.
165Riedel KD et al. Simultaneous determination of hypericin and hyperforin in human plasma with liquid chromatography-tandem mass spectrometry. J Chromatog B 2004; 813: 27–33.
166Ernst E et al. Adverse effects profile of the herbal antidepressant St John's wort (Hypericum perforatum L.). Eur J Clin Pharmacol 1998; 54: 589–594.
167Dyson R et al. The reported use and effectiveness of Hypericum (St John's wort) on affective symptoms in a depression self-help group.
Primary Care Psych 2002; 8: 99–102.
168Durán N, Song P-S. Hypericin and its photodynamic action.
Photochem Photobiol 1986; 43: 677–680.
169Brockmöller J et al. Hypericin and pseudohypericin: pharmacokinetics and effects on photosensitivity in humans. Pharmacopsychiatry 1997; 30 (Suppl. Suppl.): 94–101.
170Schempp C et al. Hypericin levels in human serum and interstitial skin blister fluid after oral single-dose and steady-state administration of
Hypericum perforatum extract (St John's wort). Skin Pharmacol Appl Skin Physiol 1999; 12: 299–304.
171Schempp CM et al. Effect of oral administration of Hypericum perforatum extract (St. John's wort) on skin erythema and pigmentation induced by UVB, UVA, visible light and solar simulated radiation. Phytother Res 2003; 17: 141–146.
172Schempp CM et al. Single-dose and steady-state administration of Hypericum perforatum extract (St John's wort) does not influence skin
sensitivity to UV radiation, visible light, and solar-simulated
S173 Pitisuttithum P et al. Int Conf AIDS 1996; 11: 285. Cited by G50 174 Schempp CM et al. Effect of topical application of Hypericum
perforatum extract (St John's wort) on skin sensitivity to solar simulated radiation. Photodermatol Photoimmunol Photomed 2000;radiation. Arch Dermatol 2001; 337: 512.
16: 125–128.
175 Cotterill JA et al. Severe phototoxic reaction to laser treatment in a patient taking St John's wort. J Cosmetic Laser Ther 2001; 3: 159–160.
176 Benner MH, Lee HJ. Med Lett 1979; 21: 29–30.
177 Barbenel DM et al. Mania in a patient receiving testosterone replacement post-orchidectomy taking St John's wort and sertraline. J Psychopharmacol 2000; 14: 84–86.
178Nierenberg AA et al. Mania associated with St John's wort. Biol Psychiatr 1999; 46: 1707–1708.
179Schneck C. St John's wort and hypomania. J Clin Psychiatr 1998; 59: 689.
180O'Breasil AM, Argouarch S. Hypomania and St John's wort. Can J Psychiatr 1998; 43: 746–747.
181Stevinson C, Ernst E. Can St John's wort trigger psychoses? Int J Clin Pharmacol Ther 2004; 42(9): 473–480.
182Bove GM. Acute neuropathy after exposure to sun in a patient treated with St John's wort. Lancet 1998; 352: 1121–1122.
183 Baillie N. Hypericum – four hypersensitivity reactions. Modern Phytother 1997; 3: 24–26.
184Patel S et al. Hypertensive crisis associated with St John's wort. Am J Med 2002; 112: 507–508.
185Siepmann M et al. The effects of St John's wort extract on heart rate variability, cognitive function and quantitative EEG: a comparison with amitriptyline and placebo in healthy men. Br J Clin Pharmacol 2002; 54: 277–282.
186Siepmann M et al. The effects of St John's wort extract and amitriptyline on autonomic responses of blood vessels and sweat glands in healthy volunteers. J Clin Psychopharmacol 2004; 24(1): 79– 82.
187Timoshanko A et al. A preliminary investigation on the acute pharmacodynamic effects of hypericin on cognitive and psychomotor performance. Behav Pharmacol 2001; 12: 635–640.
188Ferko N, Levine MAH. Evaluation of the association between St John's wort and elevated thyroid-stimulating hormone. Pharmacotherapy 2001; 21(12): 1574–1578.
189Assalian P. Sildenafil for St John-wort induced sexual dysfunction. J Sex Marital Ther 2000; 26: 357–358.
190Bhopal JS. St John's wort-induced sexual dysfunction. Can J Psychiatry 2001; 46: 456–457.
191Giese AC. Hypericism. Photochem Photobiol 1980; 5: 229–255.
192Siegers C et al. Zur Frage der Phototoxizität von Hypericum.
Nervenheilkunde 1993; 12: 320–322.
193Wilhelm K-P et al. Role of flavonoids in controlling the phototoxicity of Hypericum perforatum extracts. Phytomedicine 2001; 8(4): 306– 309.
194Johnston N. Sun trap. New Sci 1999; 24 July: 24.
195Uppsala Monitoring Centre. Vigibase, WHO Adverse Reactions database (accessed 24 October, 2005).
196Capasso R et al. Effects of the antidepressant St John's wort (Hypericum perforatum) on rat and human vas deferens contractility. J Urol 2005; 173: 2194–2197.
197Garrett BJ et al. Consumption of poisonous plants (Senecio jacobaea,
Symphytum officinale, Pteridium aquilinum, Hypericum perforatum) by rats: chronic toxicity, mineral metabolism, and hepatic drugmetabolizing enzymes. Toxicol Lett 1982; 10: 183–188.
198Newall CA et al. Herbal Medicines. A Guide for Health-care Professionals, 1st edn. London: The Pharmaceutical Press, 1996.
199Henderson L et al. St John's wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol 2002; 54: 349– 356.
200Mannel M. Drug interactions with St John's wort. Drug Saf 2004; 27 (11): 773–797.
201Eich-Hochli D et al. Methadone maintenance treatment and St John's wort – a case report. Pharmacopsychiatry 2003; 36: 35–37.
202Gordon JB. SSRIs and St John's wort: possible toxicity? Am Family Phys 1998; 57: 950–953.
203Lantz MS et al. St John's wort and antidepressant drug interactions in the elderly. J Geriat Psychiatr Neurol 1999; 12: 7–10.
204Irefin S, Sprung J. A possible case of cardiovascular collapse during anaesthesia: long-term use of St John's wort. J Clin Anaesthesiol 2000;
12:498–499.
205Crowe S, McKeating K. Delayed emergence and St John's wort. Anaesthesiology 2002; 96: 1025–1027.
206Ang-Lee MK et al. Herbal medicines and peri-operative care. JAMA 2001; 286: 208–216.
207Breckenridge A. Herbal Safety News: Important interactions between St John's wort (Hypericum perforatum) preparations and prescribed medicines. http://www.mhra.gov.uk
208Anon. CSM advice on St John's wort. Pharm J 2000; 264: 358.
209Johne A et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John's wort (Hypericum perforatum). J Clin Psychopharmacol 2002; 22(1): 46–54.
210Bauer S et al. Alterations in cyclosporin A pharmacokinetics and metabolism during treatment with St John's wort in renal transplant patients. Br J Clin Pharmacol 2003; 55: 203–211.
211Mai I et al. Hyperforin content determines the magnitude of the St John's wort–cyclosporin drug interaction. Clin Pharmacol Ther 2004;
76:330–340.

212 Mai I et al. Impact of St John's wort treatment on the pharmacokinetics of tacrolimus and mycophenolic acid in renal transplant patients. Nephrol Dial Transplant 2003; 18: 819–822.
213Mathijssen RHJ et al. Effects of St John's wort on irinotecan metabolism. J Nat Cancer Inst 2002; 94(16): 1247–1249.
214Mills E et al. Interaction of St John's wort with conventional drugs: systematic review of clinical trials. BMJ 2004; 329: 27–30.
215Johne A et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John's wort (Hypericum perforatum). Clin Pharmacol Ther 1999; 66: 338–345.
216Smith P et al. The influence of St John's wort on the pharmacokinetics and protein binding of imatinib mesylate. Pharmacotherapy 2004; 24 (11): 1508–1514.
217Frye RF et al. Effect of St John's wort on imatinib mesylate pharmacokinetics. Clin Pharmacol Ther 2004; 76: 323–329.
218Pfrunder A et al. Interaction of St John's wort with low-dose oral contraceptive therapy: a randomized controlled trial. Br J Clin Pharmacol 2003; 56: 683–690.
219Hall SD et al. The interaction between St John's wort and an oral contraceptive. Clin Pharmacol Ther 2003; 74: 525–535.
220Maurer A et al. Interaction of St John's wort extract with phenprocoumon (abstract). Eur J Clin Pharmacol 1999; 55: A22.
221Kawaguchi A et al. Drug interaction between St John's wort and quazepam. Br J Clin Pharmacol 2004; 58: 403–410.
222Sugimoto K-I et al. Different effects of St John's wort on the pharmacokinetics of simvastatin and pravastatin. Clin Pharmacol Ther 2001; 70: 518–524.
223Hebert MF et al. Effects of St John's wort (Hypericum perforatum) on tacrolimus pharmacokinetics in healthy volunteers. J Clin Pharmacol 2004; 44: 89–94.
224Tannergren C et al. St John's wort decreases the bioavailability of R- and S-verapamil through induction of the first-pass metabolism. Clin Pharmacol Ther 2004; 75: 298–309.
225Jiang X et al. Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol 2004; 57: 592–599.
226Kerb R et al. Urinary 6-beta-hydroxycortisol excretion rate is affected by treatment with hypericum extract. Eur J Clin Pharmacol 1992; 52: 607.
227Roby CA et al. St John's wort impact on CYP3A4 activity. Poster no. 129 presented at the 39th Annual Meeting of the New Clinical Drug Evaluation Unit Program, June 1999, Florida.
228Markowitz JS et al. Effect of St John's wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003; 290: 1500– 1504.
229Wang Z et al. The effects of St John's wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther 2001; 70: 317–326.
230Hennessy M et al. St John's wort increases expression of P- glycoprotein: implications for drug interactions. Br J Clin Pharmacol 2002; 53: 75–82.
231Zhou S et al. Pharmacokinetic interactions of drugs with St John's wort. J Pscychopharmacol 2004; 18: 262–276.
232Wang L-S et al. St John's wort induces both cytochrome P450 3A4catalyzed sulfoxidation and 2C19-dependent hydroxylation of omeprazole. Clin Pharmacol Ther 2004; 75: 191–197.
233Burstein AH et al. Lack of effect of St John's wort on carbamazepine pharmacokinetics in healthy volunteers. Clin Pharmacol Ther 2000; 68: 605–612.
234Morimoto T et al. Effect of St John's wort on the pharmacokinetics of theophylline in healthy volunteers. J Clin Pharmacol 2004; 44: 95–101.
235Ereshefsky B et al. Determination of St John's wort differential metabolism at CYP2D6 and CYP3A4, using dextromethorphan probe methodology. Poster no. 130 presented at the 39th Annual Meeting of the New Clinical Drug Evaluation Unit Program, June 1999, Florida.
236Gewertz N et al. Determination of the differential effects of St John's wort on the CYP1A2 and NAT2 metabolic pathways using caffeine
St John’s Wort |
569 |
probe methodology. Poster no. 131 presented at the 39th Annual Meeting of the New Clinical Drug Evaluation Unit Program, June 1999, Florida.
237 Markowitz JS et al. Effect of St John's wort (Hypericum perforatum) on cytochrome P450 2D6 and 3A4 activity in healthy volunteers. Life Sci 2000; 66(9): 133–139.
238 Wenk M et al. Effect of St John's wort on the activities of CYP1A2, CYP3A4,CYP2D6, N-acetyltransferase 2, and xanthine oxidase in healthy males and females. Br J Clin Pharmacol 2004; 57: 495–499.
239Arold G et al. No relevant interaction with alprazolam, caffeine, tolbutamide, and digoxin by treatment with a low-hyperforin St John's wort extract. Planta Med 2005; 71: 331–337.
240Johne A et al. Impact of cytochrome P-450 inhibition by cimetidine and induction by carbamazepine on the kinetics of hypericin and pseudohypericin in healthy volunteers. Eur J Clin Pharmacol 2004; 60: 617–622.
241Komoroski BJ et al. Induction and inhibition of cytochromes P450 by the St John's wort constituent hyperforin in human hepatocyte cultures. Drug Metab Disposition 2004; 32: 512–518.
242Chen Y et al. Induction of human CYP2C9 by rifampicin, hyperforin, and phenobarbital is mediated by the pregnane X receptor. J Pharmacol Exp Ther 2004; 308: 495–501.
243Cantoni L et al. Hyperforin contributes to the hepatic CYP3Ainducing effect of Hypericum perforatum extract in the mouse. Toxicol Sci 2003; 75: 25–30.
244Perloff MD et al. Saint John's wort: an in vitro analysis of P- glycoprotein induction due to extended exposure. Br J Pharmacol 2001; 134: 1601–1608.
245Tian R et al. Functional induction and de-induction of P-glycoprotein by St John's wort and its ingredients in a human colon adenocarcinoma cell line. Drug Metab Disposition 2005; 33: 547–554.
246Budzinski JW et al. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine 2000; 7: 273–282.
247Strandell J et al. An approach to the in vitro evaluation of potential for cytochrome P450 enzyme inhibition from herbals and other natural remedies. Phytomedicine 2004; 11: 98–104.
248Wang E et al. Quantitative characterization of direct P-glycoprotein inhibition by St John's wort constituents hypericin and hyperforin. J Pharm Pharmacol 2004; 56: 123–128.
249Peebles KA et al. Catalytic inhibition of human DNA topoisomerase IIa by hypericin, a naphthodianthrone from St John's wort (Hypericum perforatum). Biochemical Pharmacol 2001; 62: 1059– 1070.
250Martin-Facklam M et al. Undeclared exposure to St John's wort in hospitalised patients. Br J Clin Pharmacol 2004; 58: 437–441.
251Redvers A et al. How many patients self-medicate with St John's wort?
Psychiatric Bull 2001; 25: 254–256.
252Beckman SE et al. Consumer use of St John's wort: a survey on effectiveness, safety and tolerability. Pharmacotherapy 2000; 20: 568–
574.
253 Hindmarch M, Oakeshott P. Interactions of the oral contraceptive pill S with antibiotics and St John's wort: knowledge of female college
students. Family Prac 2002; 19(6): 708.
254Lee A et al. The safety of St John's wort (Hypericum perforatum) during breastfeeding. J Clin Psychiatry 2003; 64: 966–968.
255Grush LR et al. St John's wort during pregnancy. JAMA 1998; 280(18): 1566.
256Klier CM et al. St John's wort (Hypericum perforatum) – is it safe during breastfeeding? Pharmacopsychiatry 2002; 35: 29–30.
257Rayburn WF et al. Effect of prenatally administered hypericum (St John's wort) on growth and physical maturation of mouse offspring.
Am J Obstet Gynecol 2001; 184: 191–195.
258Gregoretti B et al. Toxicity of Hypericum perforatum (St John's wort) administered during pregnancy and lactation in rats. Toxicol Applied Pharmacol 2004; 200: 201–205.
