
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index

Lobelia
Summary and Pharmaceutical Comment
The principal constituent of lobelia is lobeline, an alkaloid with similar pharmacological properties to those of nicotine. Lobelia has previously been used in herbal preparations for the treatment of asthma and bronchitis, and in anti-smoking preparations aimed to lessen nicotine withdrawal symptoms. However, in view of its potent alkaloid constituents, excessive use of lobelia and use during pregnancy and lactation should be avoided.
Species (Family)
Lobelia inflata L. (Campanulaceae)
Synonym(s)
Indian Tobacco
Part(s) Used
Herb
L
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General Reference G6.
Figure 1 Selected constituents of lobelia.
Figure 2 Lobelia (Lobelia inflata).
Alkaloids Piperidine-type. 0.48%. Lobeline (major); others include lobelanine, lobelanidine, norlobelanine, lelobanidine, norlelobanidine, norlobelanidine and lobinine.
Other constituents Bitter glycoside (lobelacrin), chelidonic acid, fats, gum, resin and volatile oil.
Food Use
Lobelia is not generally used as a food.
Herbal Use
Lobelia is stated to possess respiratory stimulant, antasthmatic, antispasmodic, expectorant, and emetic properties. Traditionally, it has been used for bronchitic asthma, chronic bronchitis, and specifically for spasmodic asthma with secondary bronchitis. It
has also been used topically for myositis and rheumatic
nodules.(G6, G7, G8, G64)
Figure 3 Lobelia – dried drug substance (herb).
416
Dosage
Dosages for oral administration (adults) for traditional uses recommended in older and contemporary standard herbal and/or pharmaceutical reference texts are given below.
Dried herb 0.2–0.6 g as an infusion or decoction three times
daily.(G6, G7)
Liquid extract 0.2–0.6 mL (1 : 1 in 50% alcohol) three times
daily.(G6, G7)
Simple Tincture of Lobelia (BPC 1949) 0.6–2.0 mL.
Tincture Lobelia Acid 1–4 mL (1 : 10 in dilute acetic acid) three times daily.(G6, G7)
Pharmacological Actions
The pharmacological activity of lobelia can be attributed to the alkaloid constituents, principally lobeline. Lobeline has peripheral and central effects similar to those of nicotine, but is less potent. Hence, lobeline initially causes CNS stimulation followed by respiratory depression. Lobeline is also reported to possess expectorant properties.
Clinical studies
There is a lack of clinical research assessing the effects of lobelia and rigorous randomised controlled clinical trials are required.
Lobelia 417
Side-effects, Toxicity
Side-effects of lobeline and lobelia are similar to those of nicotine and include nausea and vomiting, diarrhoea, coughing, tremors and dizziness. Symptoms of overdosage are reported to include
profuse diaphoresis, tachycardia, convulsions, hypothermia, hypotension and coma, and may be fatal.(G45)
Contra-indications, Warnings
The pharmacological actions of lobeline are similar to those of nicotine.
Drug interactions None documented. However, the potential for preparations of lobelia to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered. The pharmacological activity of lobeline is similar to that of nicotine.
Pregnancy and lactation Lobelia should not be used during pregnancy or lactation.
Preparations
Proprietary multi-ingredient preparations
UK: Catarrh Tablets.
L
Marshmallow
Summary and Pharmaceutical Comment
In vitro and animal studies provide some supporting evidence for the use of marshmallow in the treatment of cough, irritation of the throat and gastric inflammation. Antibacterial and anti-inflammatory activities, effects on mucociliary transport, adhesion of polysaccharide to buccal membranes and reduction of cough are reported. However, there is a lack of clinical studies investigating the effects of marshmallow. Although no toxicity data were located, the chemistry of marshmallow and its use in foods indicate that there should not be any reason for concern regarding safety.
Species (Family)
Althaea officinalis L. (Malvaceae)
Synonym(s)
Althaea, A. taurinensis DC., A. kragujevacensis Panc.
M
Figure 1 Selected constituents of marshmallow.
Part(s) Used
Leaf, root
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
BP 2007(G84)
Complete German Commission E(G3)
ESCOP 2003(G76)
Martindale 35th edition(G85)
Ph Eur 2007(G81)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General References G2, G6 and G52.
418

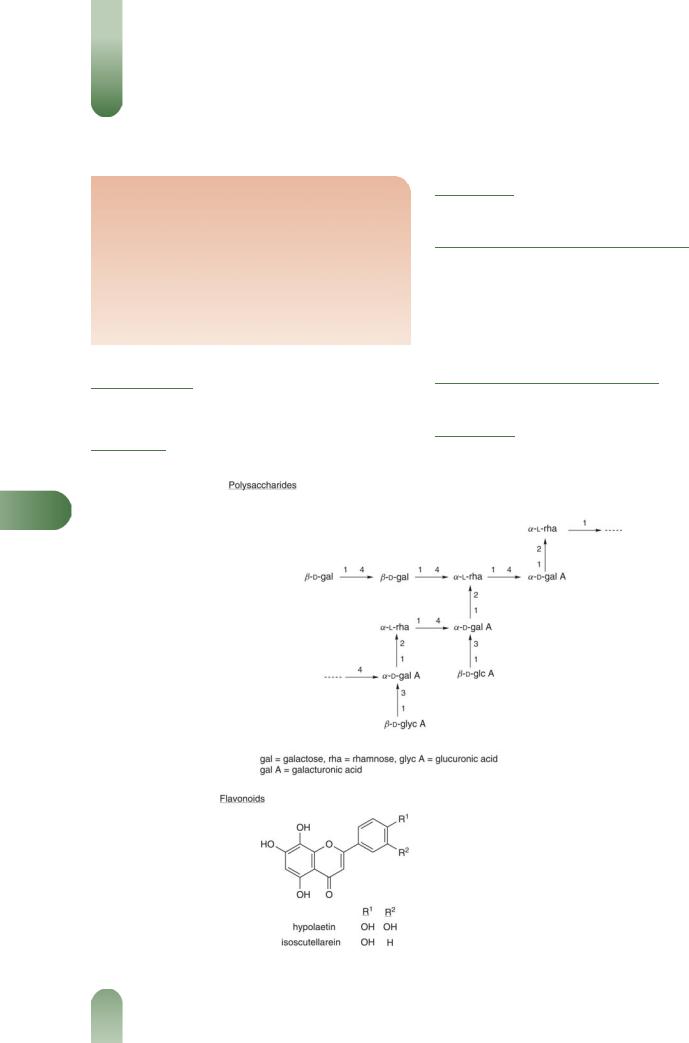
Polysaccharides Mucilage polysaccharides (5–10%), consisting
of galacturono-rhamnans, arabinans, glucans, arabinogalactans.(1,
G52)
Flavonoids Hypolaetin 8-glucoside, isoscutellarein 40-methy- lether-8-glucoside-200-sulfate.(2)
Phenolic acids Caffeic, p-coumaric, ferulic, p-hydroxybenzoic and syringic.
Other constituents Asparagine 2%, calcium oxalate, coumarins (scopoletin), pectin, starch and tannin.
Food Use
Marshmallow is listed by the Council of Europe as a natural source of food flavouring (category N2). This category indicates that marshmallow can be added to foodstuffs in small quantities,
with a possible limitation of an active principle (as yet unspecified) in the final product.(G16) Previously in the USA, marshmallow has been approved for use in foods.(G41)
Herbal Use
Marshmallow is stated to possess demulcent, expectorant,
emollient, diuretic, antilithic and vulnerary properties.(G2, G4, G6– G8, G43, G52, G54, G64) Traditionally, it has been used internally for
the treatment of respiratory catarrh and cough, peptic ulceration, inflammation of the mouth and pharynx, enteritis, cystitis, urethritis and urinary calculus, and topically for abscesses, boils and varicose and thrombotic ulcers. The German Commission E approved use of root and leaf for irritation of oral and pharyngeal mucosa and associated dry cough and root for mild inflammation of gastric mucosa.(G3) Marshmallow root is used in combination with anise fruit, eucalyptus oil, liquorice and with anise fruit, liquorice and primrose root and with anise fruit and primrose root
for catarrh of the upper respiratory tract and resulting dry cough.(G3)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in older and contemporary standard herbal and/or pharmaceutical reference texts are given below.
Dried leaf 2–5 g as an infusion three times daily;(G6, G7) 5 g.(G3)
Leaf, liquid extract 2–5 mL (1 : 1 in 25% alcohol) three times
daily.(G6, G7)
Figure 2 Marshmallow (Althaea officinalis).
Marshmallow 419
Figure 3 Marshmallow – dried drug substance (root).
Ointment 5% Powdered althaea leaf in usual ointment base three times daily.(G6, G7)
Dried root 2–5 g |
by cold extraction three times daily;(G6, G7) |
6 g.(G3) |
|
Root, liquid extract 2–5 mL (1 : 1 in 25% alcohol) three times |
|
daily.(G6, G7) |
|
Syrup of Althaea |
(BPC 1949) 2–10 mL three times daily.(G6, G7) |
Pharmacological Actions |
M |
In vitro and animal studies |
Antimicrobial activity against Pseudomonas aeruginosa, Proteus vulgaris and Staphylococcus aureus has been documented for marshmallow.(3)
The mucilage has demonstrated considerable hypoglycaemic activity in non-diabetic mice.(4)
Inhibition (17%) of mucociliary transport in ciliated epithelium
isolated from frog oesophagus was observed with 200 mL of cold macerate of marshmallow root (6.4 g/140 mL).(G52)
Marshmallow root extract is reported to stimulate phagocytosis, and to release oxygen radicals and leukotrienes from human neutrophils.(G52) In addition, release of cytokines, interleukin 6 and tumour necrosis factor from monocytes occurs, demonstrating potential anti-inflammatory and immunomodulatory effects. In mice, intraperitoneal administration of isolated polysaccharide (10 mg/kg) resulted in activity of macrophages in a carbon clearance test, and was indicative of non-specific immunomodulation.(G52) A lack of anti-inflammatory activity has been observed for marshmallow in the carrageenan-induced rat paw oedema test.(5) The anti-inflammatory effect of an ointment containing 0.05% dexamethasone was enhanced by addition of aqueous
extract of marshmallow (20%) as assessed in a rabbit ear irritancy test using UV irradiation or furfuryl alcohol.(G52)
A total extract of root and isolated polysaccharide (100 and 50 mg/kg, respectively) have been tested for their antitussive activity in unanaesthetised cats.(6) The polysaccharide gave a statistically significant decrease in the number of cough efforts from laryngopharyngeal and tracheobronchial areas. The root extract was less effective than the isolated polysaccharide.
A polysaccharide enriched extract showed moderate concentra-
tion-dependent adhesive properties in porcine buccal membranes ex vivo.(7)
420 Marshmallow
Clinical studies
There is a lack of clinical research assessing the effects of marshmallow and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
None documented. However, there is a lack of clinical safety and toxicity data for marshmallow, and further investigation of these aspects is required where therapeutic dosages are greater than the quantities ingested in foods.
Contra-indications, Warnings
Drug interactions None documented. However, the potential for preparations of marshmallow to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered. Marshmallow may delay the absorption of other medicines taken simultaneously.(G76) There is limited evidence from preclinical studies that marshmallow has hypoglycaemic activity.
Pregnancy and lactation There are no known problems with the use of marshmallow during pregnancy or lactation. However, amounts ingested should not exceed those used in foods.
Preparations
Proprietary single-ingredient preparations
MCanada: Butt-Out. France: Primadrill. Germany: Phytohustil.
Proprietary multi-ingredient preparations
Australia: Althaea Complex; Cough Relief; Garlic and Horseradish þ C Complex; Hydrastis Complex; Potassium Iodide and Stramonium Compound. Austria: Heumann's Bronchialtee; Paracodin; The Chambard-Tee; Tuscalman. Belgium: Kamfeine. Brazil: Asmatiron; Broncofenil; Bronquidex; Brontoss; Expectobron; Expectol; Iodeto de Potassio; Iodeto de
Potassio; Iol; Iolin; MM Expectorante; Peitoral Angico Pelotense; Pulmoforte. Canada: Original Herb Cough Drops; Swiss Herb Cough Drops. Chile: Paltomiel Plus; Pulmagol; Ramistos. Czech Republic: Detska Cajova Smes; Detsky Caj s Hermankem; Nontusyl; Pruduskova; Pulmoran; Species Pectorales Planta. France: Mediflor Tisane No 4 Diuretique. Germany: Heumann Bronchialtee Solubifix T; Tonsilgon. Italy: Gastrotuss. Malaysia: Horseradish Plus. Russia: Linkus (Линкас); Pansoral Teething (Пансорал Первые Зубы); Tonsilgon N (Тонзилгон Н). South Africa: Cough Elixir. Spain: Bronpul; Llantusil; Malvaliz; Natusor Broncopul; Natusor Farinol; Natusor Gastrolen; Natusor Malvasen; Pazbronquial; Senalsor. Switzerland: Malveol; Tisane pectorale et antitussive; Tisane pectorale pour les enfants; Tisane Provencale No 1; Tuscalman. UK: Antibron; Asthma & Catarrh Relief; Balm of Gilead; Chest Mixture; Herb and Honey Cough Elixir; Herbelix; Herbheal Ointment; Horehound and Aniseed Cough Mixture; Modern Herbals Cold & Catarrh; Modern Herbals Cold & Congestion; Napiers Uva Ursi Tea; Potter's Catarrh Pastilles; Sinotar; Vegetable Cough Remover. USA: Laci Le Beau Super Dieter's Tea.
References
1Blaschek W, Franz G. A convenient method for the quantitative determination of mucilage polysaccharides in Althaeae radix. Planta Med 1986; 52 (Suppl. Suppl.): 537.
2Gudej J. Flavonoids, phenolic acids and coumarins from the roots of
Althaea officinalis. Planta Med 1991; 57: 284–285.
3Recio MC et al. Antimicrobial activity of selected plants employed in the Spanish Mediterranean area Part II. Phytother Res 1989; 3: 77–80.
4Tomodo M et al. Hypoglycaemic activity of twenty plant mucilages and three modified products. Planta Med 1987; 53: 8–12.
5 Mascolo N et al. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phytother Res 1987; 1: 28–31.
6 Nos ´ova G et al. Antitussive wirkung des extraktes und der a´l
polysaccharide aus eibisch (Althaea officinalis L., var. robusta). Pharmazie 1992; 47: 224–226.
7Schmidgall J et al. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Med 2000; 66: 48–53.
Mate´
Summary and Pharmaceutical Comment
Mate´ is characterised by the xanthine constituents, which also represent the active principles. The herbal uses of mate´ can be attributed to the pharmacological actions of caffeine, which are well documented. However, there is a lack of clinical research assessing the efficacy and safety of preparations of mate´. Side-effects and warnings associated with other xanthine-containing beverages, such as tea and coffee, are applicable to mate´.
Species (Family)
Ilex paraguariensis St. Hil. (Aquifoliaceae)
Synonym(s)
Ilex, Jesuit's Brazil Tea, Paraguay Tea, St Bartholomew's Tea, Yerba Maté
Part(s) Used
Leaf
Pharmacopoeial and Other Monographs
BHP 1996(G9)
Complete German Commission E(G3)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General Reference G2.
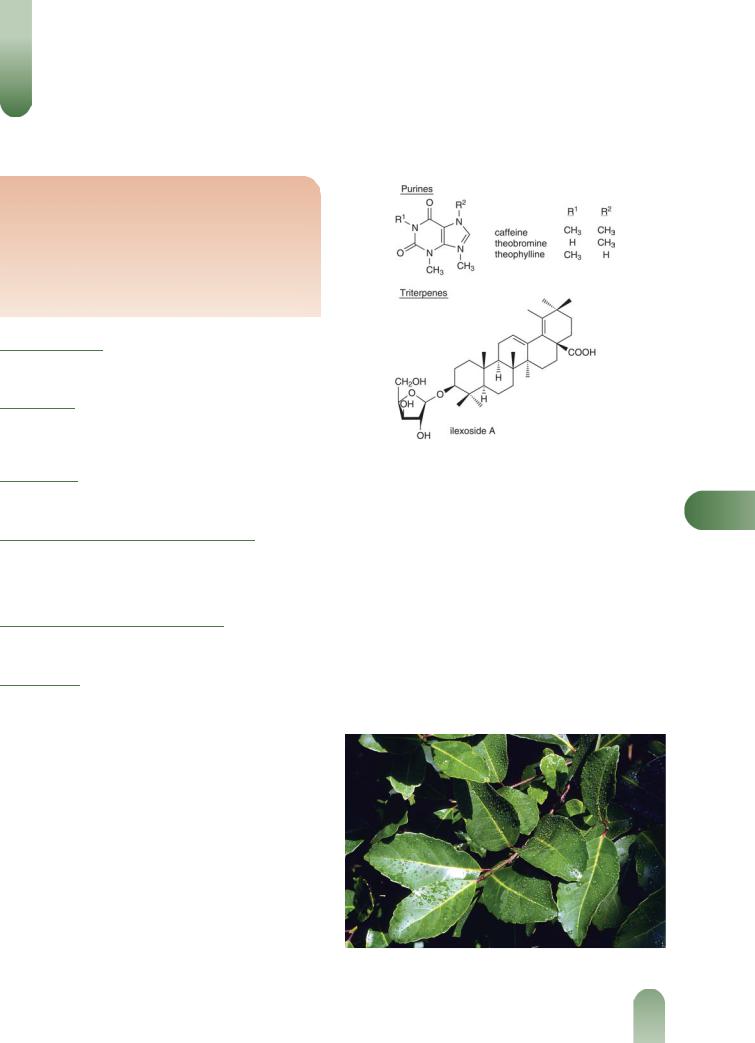
Alkaloids Xanthine-type. Caffeine 0.2–2.0%, theobromine 0.1– 0.2%, theophylline 0.05%.
Flavonoids Kaempferol, quercetin, and their glycosides, including rutin.(1)
Tannins 4–16%.
Terpenoids Ursolic acid (major), b-amyrin, ilexoside A, ilexoside B methyl ester.(2)
Other constituents Choline and trigonellin (amines), amino acids,(1) riboflavin (vitamin B2), pyridoxine (vitamin B6), niacin, pantothenic acid, vitamin C and resins.
Other Ilex species Triterpenoid saponins termed ilexsaponins B1,
B2, and B3 have been isolated from Ilex pubescens Hook. & Arn.(3)
A cyanogenetic glucoside has been isolated from Ilex aquifolium.(4)
Figure 1 Selected constituents of mate´.
Food Use |
|
M |
|
Maté is listed by the Council of Europe as a natural source of food |
|||
flavouring (category N2). This category indicates that maté can be |
|
||
added to foodstuffs in small quantities, with a possible limitation |
|
||
of an active principle (as yet unspecified) in the final product.(G16) |
|
||
Maté is commonly consumed as a beverage. It is stated to be less |
|
||
astringent than tea.(G45) Previously, maté has been listed as GRAS |
|
||
(Generally Recognised As Safe).(G65) |
|
||
Herbal Use |
|
||
|
|
|
|
Maté is stated to possess CNS-stimulant, thymoleptic, diuretic, |
|
||
antirheumatic and mild analgesic properties. Traditionally, it has |
|
||
been used for psychogenic headache and fatigue, nervous |
|
||
depression, rheumatic pains, and specifically for headache |
|
||
associated with fatigue.(G2, G7, G8, G64) |
|
||
Figure 2 Mate´ (Ilex paraguariensis).
421

422 Mate´
Figure 3 Mate´ – dried drug substance (leaf).
Dosage
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Dried leaf 2–4 g as an infusion three times daily.(G6, G7)
Liquid extract 2–4 mL (1 : 1 in 25% alcohol) three times
daily.(G6, G7)
Pharmacological Actions
MIn vitro and animal studies
In vivo hypotensive activity in rats has been reported for an aqueous extract of Ilex pubescens (commonly referred to as
maodong qing or MDQ) It was concluded that intravenous administration of MDQ releases histamine.(5)
Clinical studies
There is a lack of clinical research assessing the effects of maté and rigorous randomised controlled clinical trials are required.
The xanthine constituents, in particular caffeine, are the active principles in maté. The pharmacological actions of caffeine are well documented and include stimulation of the CNS, respiration
and skeletal muscle, in addition to cardiac stimulation, coronary dilation, smooth muscle relaxation and diuresis.(G41) Reduction of appetite has been documented for maté.(1)
In China, MDQ is used parenterally for the treatment of cardiovascular diseases (hypotensive action).(1)
Side-effects, Toxicity
Side-effects generally associated with xanthine-containing beverages include sleeplessness, anxiety, tremor, palpitations and withdrawal headache.
Veno-occlusive disease of the liver in a young woman has been attributed to the consumption of large quantities of maté over a number of years.(G45) The association between consumption of maté infusions and oesophageal cancer has been investigated in Uruguay, where oesophageal cancer constitutes a major public health problem.(6, 7) Heavy consumption was reported to elevate the relative risk of oesophageal cancer by 6.5 and 34.6 in men and
women, respectively.
The fatal dose of caffeine in humans is stated to be 10 g.(G41)
Contra-indications, Warnings
Warnings generally associated with caffeine are applicable, such as restricted intake by individuals with hypertension or a cardiac disorder.
Drug interactions None documented for maté. However, the potential for preparations of maté to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered. Warnings generally associated with caffeine are applicable.
Pregnancy and lactation It is generally recommended that caffeine consumption should be restricted during pregnancy, although conflicting results have been documented concerning the association between birth defects and caffeine consumption. In view of this, excessive consumption of maté during pregnancy should be avoided. Caffeine is excreted in breast milk, but at concentrations too low to represent a hazard to breastfed infants.(G45) As with all xanthine-containing beverages, excessive consumption of maté by breastfeeding mothers should be avoided.
References
1Ohem N, Holzl J. Some new investigations on Ilex paraguariensis – Flavonoids and triterpenes. Planta Med 1988; 54: 576.
2Inada A. Two new triterpenoid glycosides from the leaves of Ilex chinensis. Chem Pharm Bull 1987; 37: 884–885.
3 Hidaka K et al. New triterpene saponins from Ilex pubescens. Chem Pharm Bull 1987; 35: 524–529.
4Willems M. Quantification and distribution of a novel cyanogenic glycoside in Ilex aquifolium. Planta Med 1989; 55: 114.
5Yang ML, Pang PKT. The vascular effects of Ilex pubescens. Planta Med 1986; 52: 262–265.
6 Morton JF. The potential carcinogenicity of herbal tea. Environ Carcino Rev. J Environ Sci Health 1986; C4: 203–223.
7Vassallo A et al. Esophageal cancer in Uruguay: a case control study. J Natl Cancer Inst 1985; 75: 1005–1009.
