
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index
Holy Thistle
Summary and Pharmaceutical Comment
The chemistry of holy thistle is well documented and the available pharmacological data support most of the stated uses. However, there is a lack of clinical research assessing efficacy and safety of preparations of holy thistle. In view of the lack of toxicity data, excessive use of holy thistle and use during pregnancy and lactation should be avoided.
Species (Family)
HCnicus benedictus L. (Asteraceae/Compositae)
Synonym(s)
Blessed Thistle, Carbenia Benedicta, Carbenia benedicta (L.) Arcang., Carduus Benedictus, Cnicus
Part(s) Used
Herb
Pharmacopoeial and Other Monographs
BHC 1992(G6) BHP 1996(G9)
Complete German Commission E(G3) Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL(G37)
Constituents
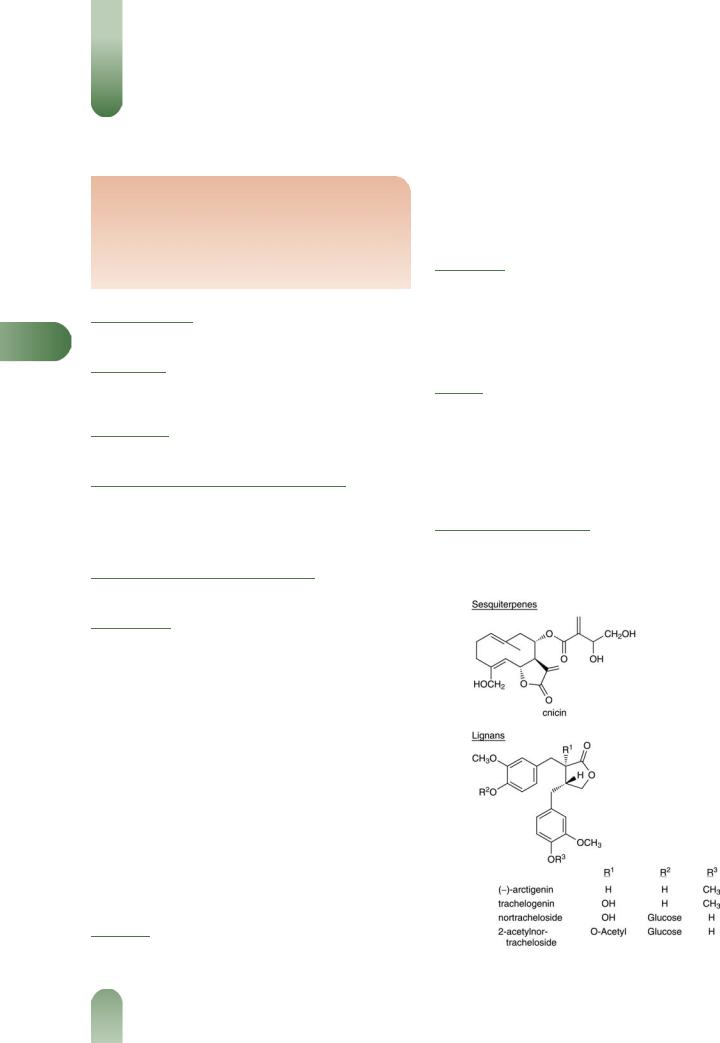
The following is compiled from several sources, including General References G2, G6, G30, G40 and G62.
Lignans Arctigenin, nortracheloside, 2-acetyl nortracheloside and trachelogenin.(1)
Polyenes Several polyacetylenes.(2)
Steroids Phytosterols (e.g. n-nonacosan, sitosterol, sitosteryl glycoside, stigmasterol).(3)
Tannins Type unspecified (8%).
Terpenoids Sesquiterpenes including cnicin 0.2–0.7%,(4) yielding salonitenolide as aglycone,(5) and artemisiifolin. Shoot and flowering head are reported to be devoid of cnicin.(4) Triterpenoids
including a-amyrenone, a-amyrin acetate, a-amyrin, multiflorenol, multiflorenol acetate and oleanolic acid.(3)
Volatile oils Many components, mainly hydrocarbons.(6)
Other constituents Lithospermic acid, mucilage, nicotinic acid and nicotinamide complex, resin.
Food Use
Holy thistle is listed by the Council of Europe as natural source of food flavouring (category N2). This category indicates that holy
thistle can be added to foodstuffs in small quantities, with a
possible limitation of an active principle (as yet unspecified) in the final product.(G16) Previously in the USA, holy thistle was
permitted for use in alcoholic beverages.(G65)
Herbal Use
Holy thistle is stated to possess bitter stomachic, antidiarrhoeal, antihaemorrhagic, febrifuge, expectorant, antibiotic, bacteriostatic, vulnerary and antiseptic properties. Traditionally, it has been used for anorexia, flatulent dyspepsia, bronchial catarrh, topically for gangrenous and indolent ulcers, and specifically for atonic dyspepsia, and enteropathy with flatulent colic.(G6, G64)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Dried flowering tops 1.5–3.0 g as an infusion three times
daily.(G6, G7)
Liquid extract 1.5–3.0 mL (1 : 1 in 25% alcohol) three times
daily.(G6, G7)
Pharmacological Actions
In vitro and animal studies
Antibacterial activity has been reported for an aqueous extract of the herb, for cnicin, and for the volatile oil.(6–9) Activity has been
Figure 1 Selected constituents of holy thistle.
352

Holy Thistle |
353 |
Figure 2 Holy thistle (Cnicus benedictus).
documented against Bacillus subtilis, Brucella abortus, Brucella bronchoseptica, Escherichia coli, Proteus species, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus faecalis. The antimicrobial activity of holy thistle has been attributed to cnicin and to the polyacetylene constituents.(9)
Cnicin has exhibited in vivo anti-inflammatory activity (carrageenan-induced rat-paw oedema test) virtually equipotent to indometacin.(4) Antitumour activity has been documented in mice against sarcoma 180 for the whole herb,(8) and against lymphoid leukaemia for cnicin;(8) cnicin has also been reported to exhibit in vitro activity against KB cells.(8) An a-methylene-g- lactone moiety is thought to be necessary for the antibacterial and antitumour activities of cnicin.(8)
Lithospermic acid is thought to be responsible for the antigonadotrophic activity documented for holy thistle.(G30) The sesquiterpene lactone constituents are stated to be bitter principles.(G62)
Tannins are generally known to possess astringent properties.
Clinical studies
There is a lack of clinical research assessing the effects of holy thistle and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
None documented. However, there is a lack of clinical safety and toxicity data for holy thistle and further investigation of these aspects is required.
The toxicity of cnicin has been studied in mice: the acute oral LD50 was stated to be 1.6–3.2 mmol/kg body weight and intraperitoneal administration was reported to cause irritation of tissue. In the writhing test, cnicin was found to cause abdominal pain with an ED50 estimated as 6.2 mmol/kg.(4)
Antitumour activity has been documented for the whole herb and for cnicin (see Pharmacological Actions; In vitro and animal studies).
Contra-indications, Warnings
None documented for holy thistle. Plants containing sesquiterpene lactones with an a-methylene-g-lactone moiety are generally considered to be allergenic, although no documented hypersensi-
Figure 3 Holy thistle – dried drug substance (herb).
tivity reactions to holy thistle were located. Holy thistle may cause |
H |
|
|
an allergic reaction in individuals with a known hypersensitivity |
|
to other members of the Compositae (e.g. chamomile, ragwort, |
|
tansy). |
|
Drug interactions None documented. However, the potential for |
|
preparations of holy thistle to interact with other medicines |
|
administered concurrently, particularly those with similar or |
|
opposing effects, should be considered. |
|
Pregnancy and lactation The safety of holy thistle has not been |
|
established. In view of the lack of toxicity data, excessive use of |
|
holy thistle during pregnancy and lactation should be avoided. |
|
Preparations
Proprietary multi-ingredient preparations
Austria: Mariazeller. Brazil: Digestron. Czech Republic:
Ungolen. Germany: Gallexier; Gastritol. Russia: Original Grosser Bittner Balsam (Оригинальный Большой Бальзам Биттнера). South Africa: Essens Amara of Groen Amara. Switzerland: Gastrosan. UK: Bio-Strath Artichoke Formula; Sure-Lax (Herbal).
References
1 Vanhaelen M, Vanhaelen-Fastré R. Lactonic lignans from Cnicus benedictus. Phytochemistry 1975; 14: 2709.
2Vanhaelen-Fastré R. Constituents polyacetyleniques de Cnicus benedictus L. Planta Med 1974; 25: 47–59.
3Ulubelen A, Berkan T. Triterpenic and steroidal compounds of Cnicus benedictus. Planta Med 1977; 31: 375–377.
4 Schneider G, Lachner I. A contribution to analytics and pharmacology of Cnicin. Planta Med 1987; 53: 247–251.
5Vanhaelen-Fastré R, Vanhaelen M. Presence of saloniténolide in
Cnicus benedictus. Planta Med 1974; 26: 375–379.
6Vanhaelen-Fastré R. Constitution and antibiotical properties of the essential oil of Cnicus benedictus. Planta Med 1973; 24: 165–175.
7 Cobb E. Antineoplastic agent from Cnicus benedictus. British Patent 1,335,181 (Cl.A61k) 24 Oct 1973, Appl.54,800/69 (via Chemical Abstracts 1975; 83: 48189j).
8Vanhaelen-Fastré R. Antibiotic and cytotoxic activities of cnicin isolated from Cnicus benedictus. J Pharm Belg 1972; 27: 683–688.
9Vanhaelen-Fastré R. Cnicus benedictus: Separation of antimicrobial constituents. Plant Med Phytother 1968; 2: 294–299.
Hops
Summary and Pharmaceutical Comment
The chemistry of hops is well documented and is characterised by the bitter acid components of the oleo-resin. Documented pharmacological activities support the herbal uses, although evidence from robust clinical studies is limited. Excessive use of hops and use during pregnancy and lactation should be avoided in view of the limited toxicity data.
Species (Family)
HHumulus lupulus L. (Cannabaceae/Moraceae)
Synonym(s)
Humulus, Lupulus
Part(s) Used
Strobile
Pharmacopoeial and Other Monographs
BHC 1992(G6) BHP 1996(G9) BP 2007(G84)
Complete German Commission E(G3) ESCOP 2003(G76)
Martindale 35th edition(G85) Ph Eur 2007(G81)
Legal Category (Licensed Products)
GSL(G37)
Constituents
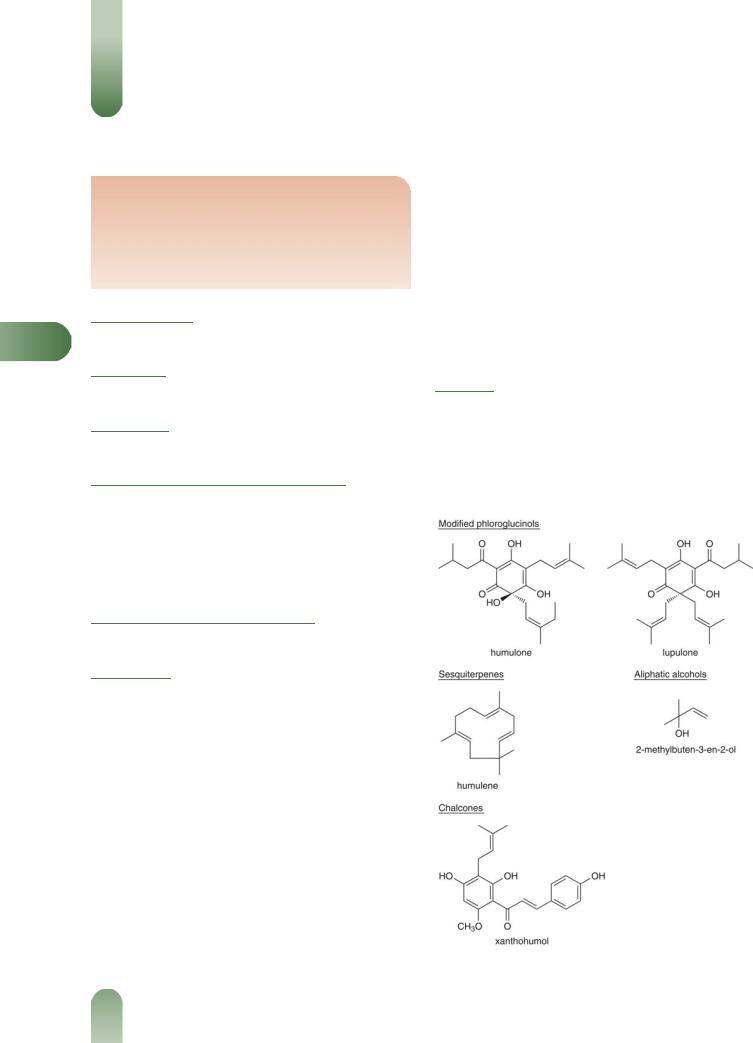
The following is compiled from several sources, including General References G2, G6 and G52.
Flavonoids Astragalin, kaempferol, quercetin, quercitrin and rutin.
Chalcones Isoxanthohumol, xanthohumol, 6-isopentenylnarin- genin, 30-(isoprenyl)-20, 4-dihydroxy-40, 60-dimethoxychalcone, 20,60-dimethoxy-4, 40-dihydroxychalcone.(1)
Oleo-resin 15-30%. Bitter principles (acylphloroglucides) in a soft and hard resin. The lipophilic soft resin consists mainly of a- acids (e.g. humulone, cohumulone, adhumulone, prehumulone, posthumulone), b-acids (e.g. lupulone, colupulone, adlupulone), and their oxidative degradation products including 2-methyl-3- buten-2-ol(2, 3, G52) The hard resin contains a hydrophilic d-resin and -resin.
Tannins 2–4%. Condensed; gallocatechin identified.(4)
Volatile oils 0.3–1.0%. More than 100 terpenoid components identified; primarily (at least 90%) b-caryophyllene, farnescene and humulene (sesquiterpenes), and myrcene (monoterpene).
Other constituents Amino acids, phenolic acids, gamma-linoleic acids, lipids and oestrogenic substances (disputed).(5)
It has been stated that only low amounts of 2-methyl-3-buten-2- ol, the sedative principle identified in hops, are present in sedative tablets containing hops.(2) However, it is thought that 2-methyl-3- buten-2-ol is formed in vivo by metabolism of the a-bitter acids and, therefore, the low amount of 2-methyl-3-buten-2-ol in a preparation may not indicate low sedative activity.(6) Interestingly, relatively high concentrations of 2-methyl-3-buten-2-ol were found in bath preparations, suggesting that high concentrations of 2- methyl-3-buten-2-ol may be achieved in both tea and bath products containing hops.(2)
Food Use
Hops are listed by the Council of Europe as a natural source of food flavouring (category N2). This category indicates that hops can be added to foodstuffs in small quantities, with a possible limitation of an active principle (as yet unspecified) in the final
product.(G16) Previously, hops has been listed as GRAS (Generally Recognised As Safe).(G65)
Figure 1 Selected constituents of hops.
354

Herbal Use
Hops are stated to possess sedative, hypnotic and topical bactericidal properties. Traditionally, they have been used for neuralgia, insomnia, excitability, priapism, mucous colitis, topically for crural ulcers, and specifically for restlessness associated with nervous tension headache and/or indigestion. The German Commission E approved use for mood disturbances such as restlessness and anxiety as well as sleep disturbances.(G3) Hops are used in combination with valerian root for nervous sleeping disorders and conditions of unrest.(G3)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in contemporary standard herbal reference texts are given below.
Dried strobile 0.5 g as an infusion two to four times daily.(G76)
Liquid extract 0.5–2.0 mL (1 : 1 in 45% alcohol) up to three times daily.(G76)
Tincture 1–2 mL (1 : 5 in 60% alcohol) up to three times daily.(G76)
Pharmacological Actions
In vitro and animal studies
Antibacterial activity, mainly against Gram-positive bacteria, has been documented for hops, and attributed to the humulone and lupulone constituents.(7) The activity of the bitter acids against Gram-positive bacteria is thought to involve primary membrane leakage. Resistance of Gram-negative bacteria to the resin acids is attributed to the presence of a phospholipid-containing outer membrane, as lupulone and humulone are inactivated by serum phospholipids.(7) Structure–activity studies have indicated the requirement of a hydrophobic molecule and a six-membered central ring for such activity.(8)
Hops |
355 |
|
The humulones and lupulones are thought to possess little |
|
|
activity towards fungi or yeasts. However, antifungal activity has |
|
|
been documented for the bitter acids towards Trichophyton, |
|
|
Candida, Fusarium and Mucor species.(9) Flavonone constituents |
|
|
have also been documented to possess antifungal activity towards |
|
|
Trichophyton and Mucor species, and antibacterial activity |
|
|
towards Staphylococcus aureus.(10) |
|
|
Antispasmodic activity has been documented for an alcoholic |
|
|
hops extract on various isolated smooth muscle preparations.(11) |
|
|
Hops have been reported to exhibit hypnotic and sedative |
|
|
properties.(G41) 2-Methyl-3-buten-2-ol, a bitter acid degradation |
|
|
product, has been identified as a sedative principle in hops.(2, 3) 2- |
|
|
Methyl-3-buten-2-ol has been shown to possess narcotic proper- |
|
|
ties in mice and motility depressant activity in rats, with the latter |
|
|
not attributable to a muscle-relaxant effect.(12) It has also been |
|
|
suggested that isovaleric acid residues present in hops may |
|
|
contribute towards the sedative action. In mice, hops extract |
|
|
administered intraperitoneally (100, 250, 500 mg/kg) 30 minutes |
H |
|
prior to a series of behavioural tests, resulted in a dose-dependent |
||
suppression of spontaneous locomotor at doses of 250 mg/kg for |
|
|
up to one hour.(13) The time for mice to be able to remain on a |
|
|
rota rod was decreased by 59% and 65% at doses of 250 mg/kg |
|
|
and 500 mg/kg, respectively. The time of onset of convulsions and |
|
|
survival time after administration of pentylenetetrazole (100 mg/ |
|
|
kg) was significantly lengthened. Hops extract |
(35 mg/kg, |
|
intraperitoneal administration) produced a dose-dependent increase in sleeping time in mice treated with pentobarbitol. An antinociceptive effect was noted by increased latency of licking forepaws in hotplate tests and hypothermic activity observed from
a time-dependent fall of rectal temperature at a dose of 500 mg/
kg.(13)
Hops have previously been reported to possess oestrogenic constituents.(5) However, when a number of purified components, including the volatile oil and the bitter acids, were examined using the uterine weight assay in immature female mice, no oestrogenic activity was found.(5)
Clinical studies
Clinical research assessing the effects of hops is limited and rigorous randomised controlled clinical trials are required.
Clinical studies have generally assessed hops given in combination with one or more additional herbs. For example, hops has been reported to improve sleep disturbances when given in combination with valerian (see Valerian, Clinical studies).(14)
Figure 2 Hops (Humulus lupulus). |
Figure 3 Hops – dried drug substance (strobile). |

356 Hops
Hops, in combination with chicory and peppermint, has been documented to relieve pain in patients with chronic cholecystitis (calculous and non-calculous).(15) A herbal product containing a mixture of plant extracts, including hops and uva-ursi, and alphatocopherol acetate was reported to improve irritable bladder and urinary incontinence.(16) However, these observations require confirmation in robust clinical studies.
Side-effects, Toxicity
There is a lack of clinical safety and toxicity data for hops and further investigation of these aspects is required.
Respiratory allergy caused by the handling of hop cones has been documented;(17) a subsequent patch test using dried, crushed flowerheads proved negative. Positive patch test reactions have been documented for fresh hop oil, humulone, and lupulone.
Myrcene, present in the fresh oil but readily oxidised, was concluded to be the sensitising agent in the hop oil.(G51) Contact
Hdermatitis to hops has long been recognised(G51) and is attributed to the pollen.(G41)
Small doses of hops are stated to be non-toxic.(G42) Large doses administered to animals by injection have resulted in a soporific
effect followed by death, with chronic administration resulting in weight loss before death.(G39)
Contra-indications, Warnings
Allergic reactions have been reported for hops, although only following external contact with the herb and oil.
Drug interactions None documented. However, the potential for preparations of hops to interact with other medicines administered concurrently, particularly those with similar or opposing
effects, should be considered. There are some conflicting data on the oestrogenic activity of hops,(5) although 8-prenylnaringenin,
documented as a constituent of hops, has been shown to have oestrogenic activity in preclinical studies.(G76) Concern has been expressed that herbs with oestrogenic effects may stimulate breast
cancer growth and oppose action of competitive oestrogen receptor antagonists such as tamoxifen.(18) However, this requires confirmation.
Pregnancy and lactation In vitro antispasmodic activity on the uterus has been documented. In view of this and the lack of toxicity data, the use of hops during pregnancy and lactation should be avoided.
Preparations
Proprietary single-ingredient preparations
Russia: Novo-Passit (Ново-Пассит). Switzerland: Klosterfrau
Nervenruh Dragees.
Proprietary multi-ingredient preparations
Australia: Extralife Sleep-Care; Humulus Compound; Natural Deep Sleep; Pacifenity; Passiflora Complex; Passionflower Plus; Prosed-X; Relaxaplex. Austria: Baldracin; Baldrian AMA; Hova; Montana; Nervenruh; Nerventee St Severin; Sedadom; Wechseltee St Severin. Canada: Herbal Sleep Aid; Herbal Sleep Well. Chile: Valupass. Czech Republic: Baldracin; Detsky Caj s Hermankem; Fytokliman Planta; Novo-Passit; Sanason; SchlafNerventee N; Species Nervinae Planta; Valofyt Neo; Visinal.
France: Nostress. Germany: Ardeysedon; Avedorm duo; Baldrian-Dispert Nacht; Baldriparan N Stark; Biosedon; Boxocalm; Cefasedativ; Dormeasan; Dormoverlan; Gutnacht; Ilja Rogoff; Klosterfrau Beruhigungs Forte; Kytta-Sedativum; Leukona-Beruhigungsbad; Luvased; Moradorm S; Nervendragees; Nervenkapseln; Nervinfant N; Nervoregin forte; Pascosedon; Schlafund Nerventee; Sedacur; Sedaselect D; Selon; Sensinerv forte; Valdispert comp; Valeriana mild; Valverde Baldrian Hopfen bei Einschlafstorungen und zur Beruhigung; Vivinox Day. Hungary: Hova. Israel: Nerven-Dragees. Italy: Emmenoiasi. Mexico: Nervinetas. Russia: Doppelherz Vitalotonik (Доппельгерц Виталотоник); Sanason (Санасон). South Africa: Avena Sativa Comp. Switzerland: Baldriparan; Dormeasan; Dragees pour le coeur et les nerfs; Dragees pour le sommeil; Dragees sedatives Dr Welti; Hova; Hyperiforce comp; Nervinetten; ReDormin; Relaxo; Soporin; Tisane calmante pour les enfants; Tisane pour le sommeil et les nerfs; Valverde Coeur; Valverde Sommeil; Zeller Sommeil. UK: Anased; Avena Sativa Comp; Boots Alternatives Sleep Well; Boots Sleepeaze Herbal Tablets; Calmanite Tablets; Fenneherb Newrelax; Gerard House Serenity; Gerard House Somnus; HRI Calm Life; HRI Night; Kalms Sleep; Kalms; Napiers Sleep Tablets; Napiers Tension Tablets; Natrasleep; Newrelax; Nodoff; Nytol Herbal; Quiet Days; Quiet Life; Quiet Nite; Quiet Tyme; Relax Bþ; Sleepezy; Slumber; Sominex Herbal; Stressless; Unwind Herbal Nytol; Valerina Night-Time; Ymea. Venezuela: Lupassin; Nervinetas.
References
1Song-San S et al. Chalcones from Humulus lupulus. Phytochemistry
1989; 28: 1776–1777.
2 Hänsel R et al. The sedative-hypnotic principle of hops. 3. Communication: Contents of 2-methyl-3-butene-2-ol in hops and hop preparations. Planta Med 1982; 45: 224–228.
3Wohlfart R et al. Detection of sedative–hypnotic hop constituents, V: Degradation of humulones and lupulones to 2-methyl-3-buten-2-ol, a hop constituent possessing sedative-hypnotic activity. Arch Pharm
(Weinheim) 1982; 315: 132–137.
4 Gorissen H et al. Separation and identification of (þ)-gallocatechin in hops. Arch Int Physiol Biochem 1968; 76: 932–934.
5Fenselau C, Talalay P. Is oestrogenic activity present in hops? Food Cosmet Toxicol 1973; 11: 597–603.
6Hänsel R, Wohlfart R. Narcotic action of 2-methyl-3-butene-2-ol contained in the exhalation of hops. Z Naturforsch 1980; 35: 1096–
1097.
7Teuber M, Schmalreck AF. Membrane leakage in Bacillus subtilis 168 induced by the hop constituents lupulone, humulone, isohumulone and humulinic acid. Arch Mikrobiol 1973; 94: 159–171.
8 Schmalreck AF et al. Structural features determining the antibiotic potencies of natural and synthetic hop bitter resins, their precursors and derivatives. Can J Microbiol 1975; 21: 205–212.
9 Mizobuchi S, Sato Y. Antifungal activities of hop bitter resins and related compounds. Agric Biol Chem 1985; 49: 399–405.
10Mizobuchi S, Sato Y. A new flavanone with antifungal activity isolated from hops. Agric Biol Chem 1984; 48: 2771–2775.
11Caujolle F et al. Spasmolytic action of hop (Humulus lupulus). Agressologie 1969; 10: 405–10.
12Wohlfart R et al. The sedative-hypnotic principle of hops. 4. Communication: Pharmacology of 2-methyl-3-buten-2-ol. Planta Med 1983; 48: 120–123.
13Lee KM et al. Effects of Humulus lupulus extract on the central nervous system in mice. Planta Med 1993; 59: A691.
14Müller-Limmroth W, Ehrenstein W. Untersuchungen über die Wirkung von Seda-Kneipp auf den Schlaf schlafgestörter Menschen. Med Klin 1977; 72: 1119–1125.
15Chakarski I et al. Clinical study of a herb combination consisting of
Humulus lupulus, Cichorium intybus, Mentha piperita in patients

|
|
Hops |
357 |
with chronic calculous and non-calculous cholecystitis. Probl Vatr |
17 |
Newmark FM. Hops allergy and terpene sensitivity: An occupational |
|
Med 1982; 10: 65–69. |
|
disease. Ann Allergy 1978; 41: 311–312. |
|
16 Lenau H et al. Wirksamkeit und Verträglichkeit von Cysto Fink bei |
18 |
Baxter KB, ed. Stockley's Drug Interactions, 7th edn. London: |
|
Patienten mit Reizblase und/oder Harninkontinenz. Therapiewoche |
|
Pharmaceutical Press, 2006. |
|
1984; 34: 6054. |
|
|
|
|
|
|
|
H
