
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index

Celery
C
Summary and Pharmaceutical Comment
Celery fruit should not be confused with the commercial celery stem, which is commonly eaten as a food. The chemistry of celery fruit is well studied and the phototoxic furanocoumarin constituents are well documented. Phototoxicity appears to be associated with the handling of the celery stems, especially diseased plant material. Limited scientific evidence is available to justify the herbal uses of celery, although bacteriostatic activity has been documented for the oil. Celery fruit should be used cautiously in view of the documented allergic reactions.
Species (Family)
Apium graveolens L. (Apiaceae/Umbelliferae)
Synonym(s)
Apii Fructus, Celery Fruit, Celery Seed, Smallage, Wild Celery
Part(s) Used
Fruit
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General References G2, G6, G48 and G58.
Flavonoids Apigenin, apiin, isoquercitrin and others.(1)
Coumarins Apigravin, apiumetin, apiumoside, bergapten, celerin, celereoside, isoimperatorin, isopimpinellin, osthenol, rutaretin, seselin, umbelliferone and 8-hydroxy-5-methoxypsora- len.
Low concentrations (not exceeding 1.3 ppm) of furanocoumarins have been identified in commercial celery,(10) although
concentrations are reported to rise considerably in diseased stems.(11)
Volatile oils 2–3%. Many components including limonene (60%)
and selenine (10–15%), and various sesquiterpene alcohols (1– 3%), e.g. a-eudesmol and b-eudesmol, santalol.(12, 13) Phthalide
compounds, 3-n-butyl phthalide and sedanenolide, provide the characteristic odour of the oil (presence of sedanolide and sedanonic anhydride disputed).(14, 15)
Other constituents Choline ascorbate,(16) fatty acids (e.g linoleic, myristic, myristicic, myristoleic, oleic, palmitic, palmitoleic, petroselinic and stearic acids).
Figure 1 Selected constituents of celery.
Food Use
Celery is listed by the Council of Europe as a natural source of food flavouring (category N2). This category indicates that celery can be added to foodstuffs in small quantities, with a possible
limitation of an active principle (as yet unspecified) in the final product.(G16) Celery stem (not the fruit) is commonly used in
foods. Previously, celery seed has been listed as GRAS (Generally Recognised As Safe).(G41)
146

Herbal Use
Celery is stated to possess antirheumatic, sedative, mild diuretic and urinary antiseptic properties. It has been used for arthritis, rheumatism, gout, urinary tract inflammation, and specifically for rheumatoid arthritis with mental depression.(G2, G6, G7, G8, G64)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in older standard herbal reference texts are given below.
Dried fruits 0.5–2.0 g as a decoction 1 : 5 three times daily.(G7)
Liquid extract 0.3–1.2 mL (1 : 1 in 60% alcohol) three times daily.(G7)
Liquid Extract of Celery (BPC 1934) 0.3–1.2 mL.
Pharmacological Actions
In vitro and animal studies
In mice, sedative and antispasmodic activities have been documented for the phthalide constituents.(17, G22) Celery seed oil has been reported to exhibit bacteriostatic activity against
Bacillus subtilis, Vibrio cholerae, Staphylococcus aureus, Staphylococcus albus, Shigella dysenteriae, Corynebacterium diphtheriae, Salmonella typhi, Streptococcus faecalis, Bacillus pumilus, Streptococcus pyogenes and Pseudomonas solanacearum.(9) No activity was observed against Escherichia coli, Sarcina lutea or
Pseudomonas aeruginosa.
Apigenin has exhibited potent antiplatelet activity in vitro, inhibiting the aggregation of rabbit platelets induced by collagen, ADP, arachidonic acid and platelet-activating factor (PAF), but not that induced by thrombin or ionophore A23187.(18)
Studies with celery plant extracts have demonstrated antiinflammatory activity in the mouse ear test and against carrageenan-induced rat paw oedema,(19) and a hypotensive effect
Celery |
147 |
|
in rabbits and dogs after intravenous administration.(G41) In |
|
|
addition, hypoglycaemic activity has been documented.(G22) |
|
|
Celery juice has been reported to exhibit choleretic activity and |
|
|
the phthalide constituents are stated to possess diuretic activity.(13) |
|
|
Clinical studies |
|
C |
There is a lack of clinical research assessing the effects of celery |
||
fruit and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
None documented. However, there is a lack of clinical safety and toxicity data for celery fruit and further investigation of these aspects is required.
Photosensitivity reactions have been reported as a result of external contact with celery stems.(20, 21, G51) These reactions have been attributed to the furanocoumarin constituents which are known to possess photosensitising properties.(11, 22) The concen-
trations of these compounds are reported to increase considerably in diseased celery stems.(11, 22) It is thought that psoralen, the most
potent phototoxic furanocoumarin, acts as a transient precursor for other furanocoumarins and does not accumulate in celery.(5, 11)
Instances of allergic and anaphylactic reactions to celery have also been documented(23) following oral ingestion of the stems.(24)
Celery allergy is reported to be mediated by IgE antibodies and an association between pollen and celery allergy has been postulated,
Figure 3 Celery – dried drug substance (root).
Figure 2 Celery (Apium graveolens). |
Figure 4 Celery – dried drug substance (fruit). |

148 Celery
although the common antigen has not been determined.(25) Crosssensitivities to celery have been documented in patients with existing allergies to dandelion and wild carrot.(G51)
Acute LD50 values (rats, by mouth; rabbits, dermal) have been reported as greater than 5 g/kg body weight.(26) Celery seed oil is
Cstated to be non-irritant, non-phototoxic and non-sensitising in
humans.(26, G58)
Contra-indications, Warnings
Celery fruit contains phototoxic compounds, furanocoumarins, which may cause photosensitive reactions. Celery fruit may precipitate allergic reactions, particularly in individuals with existing plant, pollen or food allergies. Diseased celery stems (indicated by a browning of the stem) should not be ingested.
Drug interactions None documented. However, the potential for preparations of celery to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered.
Pregnancy and lactation Celery fruit is reputed to affect the menstrual cycle and to be abortifacient.(G30) Uterine stimulant activity has been documented for the oil,(G22, G30) and the use of celery fruits is contra-indicated during pregnancy.(G49) This does not refer to celery stems that are commonly ingested as a food, although excessive consumption should be avoided. It is not known whether constituents of celery fruit appear in breast milk.
Preparations
Proprietary multi-ingredient preparations
Australia: Arthritic Pain Herbal Formula 1; Boswellia Complex; Devils Claw Plus; Fluid Loss; Guaiacum Complex; Lifesystem Herbal Formula 1 Arthritic Aid. Canada: Herbal Diuretic. India: Flexi-muv. Malaysia: Celery Plus. UK: Mixed Vegetable Tablets; Modern Herbals Rheumatic Pain; Napiers Backache Tea; Rheumatic Pain; Rheumatic Pain Tablets; Sciatica Tablets; Vegetex.
References
1Garg SK et al. Glucosides of Apium graveolens. Planta Med 1980; 38: 363–365.
2Garg SK et al. Apiumetin – a new furanocoumarin from the seeds of
Apium graveolens. Phytochemistry 1978; 17: 2135–2136.
3Garg SK et al. Celerin, a new coumarin from Apium graveolens. Planta Med 1980; 38: 186–188.
4Garg SK et al. Minor phenolics of Apium graveolens seeds.
Phytochemistry 1979; 18: 352.
5Dall'Acqua et al. Biosynthesis of O-alkylfurocoumarins. Planta Med 1975; 27: 343–348.
6 Garg SK et al. Apiumoside, a new furanocoumarin glucoside from the seeds of Apium graveolens. Phytochemistry 1979; 18: 1764–1765.
7 Garg SK et al. Coumarins from Apium graveolens seeds. Phytochemistry 1979; 18: 1580–1581.
8 Innocenti G et al. Investigations of the content of furocoumarins in
Apium graveolens and in Petroselinum sativum. Planta Med 1976; 29: 165–170.
9 Kar A, Jain SR. Investigations on the antibacterial activity of some Indian indigenous aromatic plants. Flavour Industry 1971; February.
10Beier RC et al. Hplc analysis of linear furocoumarins (psoralens) in healthy celery (Apium graveolens). Food Chem Toxicol 1983; 21: 163– 165.
11Chaudhary SK et al. Increased furocoumarin content of celery during storage. J Agric Food Chem 1985; 33: 1153–1157.
12Fehr D. Untersuchung über aromastoffe von sellerie (Apium graveolens L.). Pharmazie 1979; 34: 658–662.
13Stahl E. Drug Analysis by Chromatography and Microscopy. Ann Arbor, Michigan: Ann Arbor Science, 1973.
14Bjeldanes LF, Kim I-S. Phthalide components of celery essential oil. J Org Chem 1977; 42: 2333–2335.
15Bos R et al. Composition of the volatile oils from the roots, leaves and fruits of different taxa of Apium graveolens. Planta Med 1986; 52: 531.
16Kavalali G, Akcasu A. Isolation of choline ascorbate from Apium graveolens. J Nat Prod 1985; 48: 495.
17Gijbels MJM et al. Phthalides in roots of Apium graveolens, A. graveolens var. rapaceum. Bifora testiculata and Petroselinum crispum var. tuberosum. Fitoterapia 1985; 56: 17–23.
18Teng CM et al. Inhibition of platelet aggregation by apigenin from
Apium graveolens. Asia Pac J Pharmacol 1988; 1: 85–89.
19Lewis DA et al. The anti-inflammatory activity of celery Apium graveolens L. (Fam. Umbelliferae). Int J Crude Drug Res 1985; 23: 27– 32.
20Berkley SF et al. Dermatitis in grocery workers associated with high natural concentrations of furanocoumarins in celery. Ann Intern Med 1986; 105; 351–355.
21Austad J, Kavli G. Phototoxic dermatitis caused by celery infected by
Sclerotinia sclerotiorum. Contact Dermatitis 1983; 9: 448–451.
22Ashwood-Smith MJ et al. Mechanisms of photosensitivity reactions to diseased celery. BMJ 1985; 290: 1249.
23Déchamp C et al. Choc anaphylactique au céleri et sensibilisation à l'ambroisie et à l'armoise. Allergie croisée ou allergie concomitante? Presse Med 1984; 13: 871–874.
24Forsbeck M, Ros A-M. Anaphylactoid reaction to celery. Contact Dermatitis 1979; 5: 191.
25Pauli G et al. Celery sensitivity: clinical and immunological correlations with pollen allergy. Clin Allergy 1985; 15: 273–279.
26Opdyke DLJ. Celery seed oil. Food Cosmet Toxicol 1974; 12: 849–850.
Centaury
Summary and Pharmaceutical Comment
There is little published information specifically concerning Centaurium erythraea. Bitter components support the traditional use of centaury as an appetite stimulant, although it is said to be less active than comparable bitter herbs, such as gentian. In view of the lack of pharmacological and toxicological data, excessive use should be avoided.
Species (Family)
Centaurium erythraea Rafn. (Gentianaceae)
Synonym(s)
Centaurium minus auct., C. minus auct. subsp. minus, C. umbellatum Gilib., Common Centaury, Erythraea centaurium
(L.) Pers. subsp. centaurium
Part(s) Used
Herb
Pharmacopoeial and Other Monographs
BHP 1996(G9)
BP 2007(G84)
Complete German Commission E(G3)
ESCOP 2003(G76)
Martindale 35th edition(G85)
Ph Eur 2007(G81)
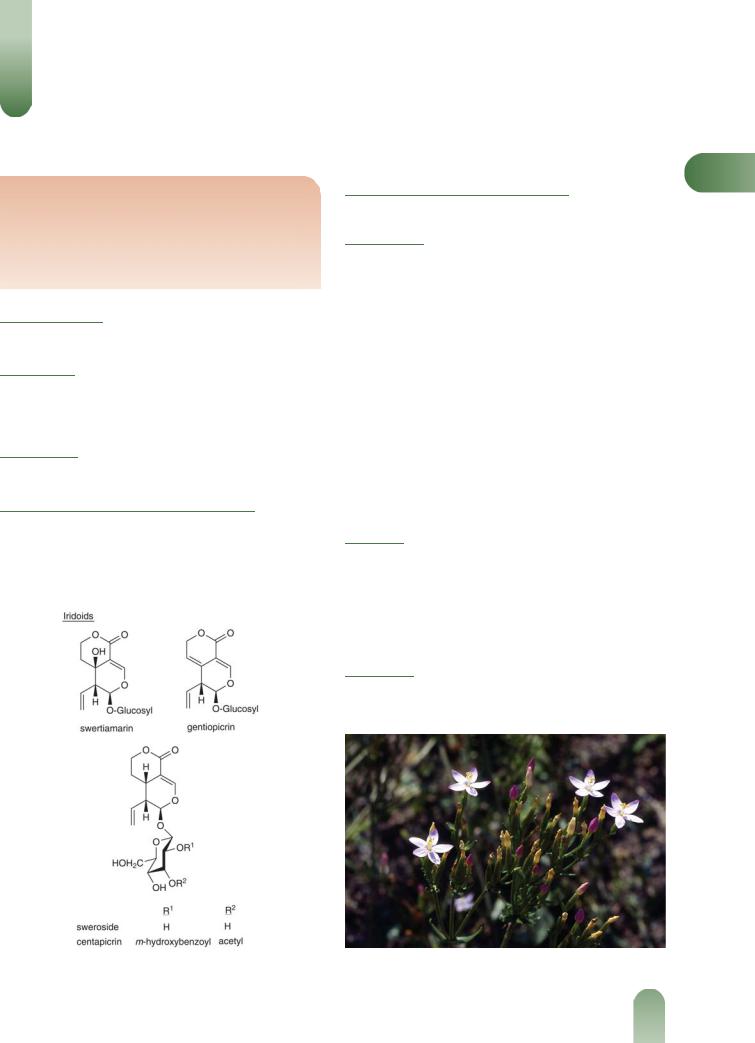
Figure 1 Selected constituents of centaury.
C
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General Reference G2.
Acids Phenolic. Protocatechuic, m- and p-hydroxybenzoic, vanillic, syringic, p-coumaric, ferulic, sinapic and caffeic, hydroxyterephthalic and 2,5-dihydroxyterephthalic acids among others.
Alkaloids Pyridine-type. Traces of gentianine, gentianidine, gentioflavine and others.
Monoterpenoids Iridoids (bitters).(1, 2) Gentiopicroside (about 2%) as major, others include centapicrin, gentioflavoside, sweroside and swertiamarin; intensely bitter m-hydroxybenzoylesters of sweroside and catapicrin.
Triterpenoids Includes a- and b-amyrin, erythrodiol, crataegolic acid, oleanolic acid and sitosterol.
Xanthones Highly methylated xanthones, including eustomin and 8-demethyleustomin.
Other constituents Flavonoids, fatty acids, alkanes and waxes.
Food Use
Centaury is listed by the Council of Europe as a natural source of food flavouring (category N2). This category indicates that centaury can be added to foodstuffs in small quantities, with a possible limitation of an active principle (as yet unspecified) in the final product.(G16) Previously, the bitter properties of centaury were utilised in alcoholic and non-alcoholic beverages with maximum permitted doses between 0.0002% and 0.0008%.(G41)
Herbal Use
Centaury is reputed to act as a bitter, aromatic and stomachic. Traditionally, it has been used for anorexia and dyspepsia.(G2, G7,
G52)
Figure 2 Centaury (Centaurium erythraea).
149

150 Centaury
C
Figure 3 Centaury – dried drug substance (herb).
Dosage
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Herb 2–4 g as an infusion three times daily.(G7)
Liquid extract 2–4 mL (1 : 1 in 25% alcohol) three times daily.(G7)
Pharmacological Actions
In vitro and animal studies
Anti-inflammatory activity has been documented in two rat models; subchronic inflammation (air pouch granuloma and polyarthritis) test,(3) and the carrageenan rat paw oedema test (19% compared to 45% with indometacin).(4) Antipyretic activity has also been exhibited by a centaury extract against experimentally induced hyperthermia in rats, although pretreatment with
the extract did not prevent hyperthermia;(3) antipyretic activity is stated to be due to the phenolic acid constituents.(G45) In the same
study, no analgesic activity could be demonstrated in mice (writhing syndrome and hotplate models).(3) Gentiopicroside (30 mg/kg/day intraperitoneally) inhibited tumour necrosis factor (TNF) production in carbon tetrachloride-induced and bacillus
Calmette–Guérin/lipopolysaccharide-induced models of hepatic injury in mice.(G52)
In rats, anticholinesterase activity has been demonstrated for swertiamarin in a dose-dependent manner following oral administration, demonstrated by inhibition of carbachol-induced contraction of proximal colon.(G52) In mice, gentianine has central nervous system (CNS)-depressant activity at oral doses of 30 mg/ kg, demonstrated by inhibition of spontaneous movement and prolonged hexobarbital-induced sleeping time.(G52) Anti-ulcero-
genic and inhibitory gastric secretion in rats (100 mg/kg) have been shown for gentianine.(G52)
Clinical studies
There is a lack of clinical research assessing the effects of centaury and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
There is a lack of clinical safety and toxicity data for centaury and further investigation of these aspects is required.
An alcoholic extract of centaury (200 mL/plate) was antimutagenic in Salmonella typhimurium strains TA8 and TA100.(G52)
Contra-indications, Warnings
Centaury is contra-indicated for individuals with peptic ulcers.(G52)
Drug interactions None documented. However, the potential for preparations of centaury to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered.
Pregnancy and lactation The safety of centaury taken during pregnancy has not been established. In view of the lack of toxicity data, use of centaury during pregnancy and lactation should be avoided.
Preparations
Proprietary single-ingredient preparations
Czech Republic: Nat Zemezluce.
Proprietary multi-ingredient preparations
Austria: Montana N; China-Eisenwein; Eryval; Magentee St Severin; Mariazeller. Czech Republic: Naturland Grosser Swedenbitter; Stomaran. France: Diacure; Tisane Hepatique de Hoerdt. Germany: Amara-Tropfen; Canephron; Stullmaton. Russia: Canephron N (Канефрон Н); Herbion Drops for the Stomach (Гербион Желудочные Капли); Original Grosser Bittner Balsam (Оригинальный Большой Бальзам Биттнера). South Africa: Amara; Clairo. Spain: Natusor Hepavesical; Odisor. Switzerland: Gastrosan; Tisane pour l'estomac.
References
1 Van der Sluis WG, Labadie RP. Onderzok naar en van secoiridoid glucosiden en zanthonen in het gescglacht Centaurium. Pharm Weekbl 1978; 113: 21–32.
2Van der Sluis WG, Labadie RP. Secoiridoids and xanthones in the genus Centaurium. Part 3. Decentapicrins A, B and C, new m- hydroxybenzoyl esters of sweroside from Centaurium littorale. Planta
Med 1981; 41: 150–160.
3 Berkan T et al. Antiinflammatory, analgesic, and antipyretic effects of an aqueous extract of Erythraea centaurium. Planta Med 1991; 57: 34– 37.
4Mascolo N et al. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phytother Res 1987; 1: 28–31.
