
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index
Eyebright
Summary and Pharmaceutical Comment
Limited information is available regarding the constituents of eyebright and it is unclear which Euphrasia species is most
Ecommonly utilised. In addition, eyebright is also used as a common name for plants other than Euphrasia species. Little scientific information was found to justify the reputed herbal uses, although tannin constituents would provide an astringent effect. The use of home-made preparations for ophthalmic purposes should be avoided. Little is known regarding the toxicity of eyebright and, in view of the reported presence of unidentified alkaloids, it should be used with caution and excessive doses and prolonged treatment should be avoided.
Species (Family)
Euphrasia officinalis L. (Scrophulariaceae)
Other Euphrasia species, including E. rostkoviana Hayne, are used and these may differ in their chemical constituents. The genus Euphrasia consists of around 450 Euphrasia species and their wild hybrids and these are difficult to identify botanically.
Synonym(s)
Euphrasia
Part(s) Used
Herb
Pharmacopoeial and Other Monographs
BHP 1983(G7)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
Eyebright is not included in the GSL.(G37)
Constituents
The following is compiled from several sources, including General References G2, G40 and G75.
Unless otherwise stated, constituents listed are for E. officinalis.
Acids Caffeic acid, ferulic acid.(1)
Alkaloids Unidentified tertiary alkaloids, choline, steam volatile bases.(1)
Amino acids Glycine, leucine and valine.
Flavonoids Four compounds (unidentified). Quercetin and rutin stated to be absent.(1) Quercetin, quercitrin and rutin have been documented for E. rostkoviana.
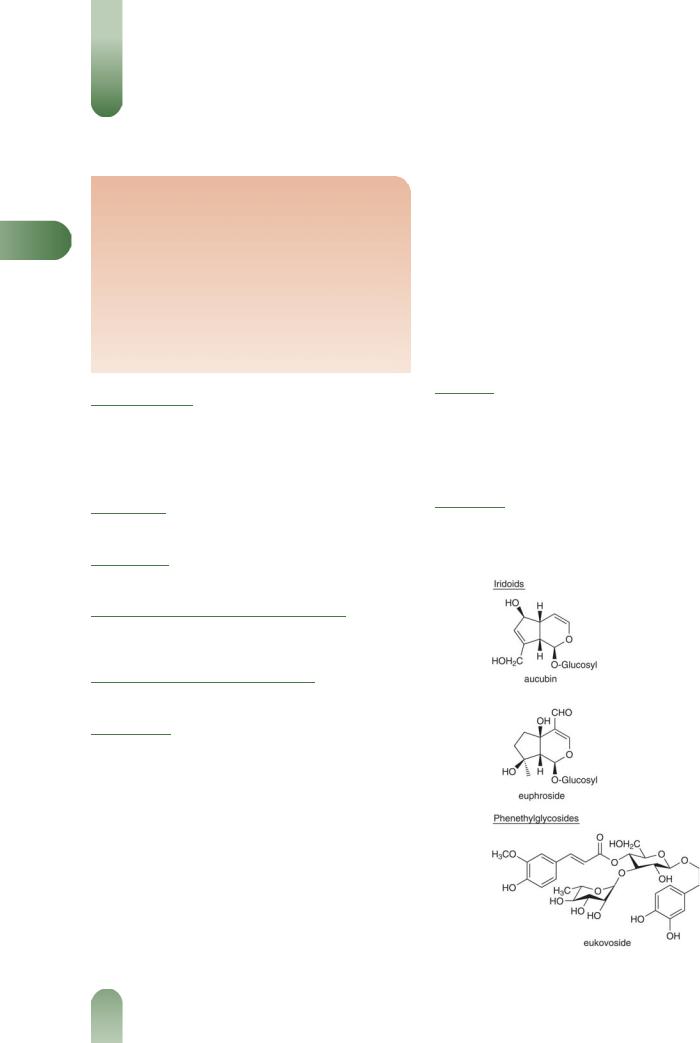
Iridoids Aucubin 0.05%. Additional glycosides have been reported for related Euphrasia species including catalpol, euphro-
side, eurostoside, geniposide, ixoroside and mussaenoside for E. rostkoviana.(2–5)
Phenethyl glycosides Dehydroconiferyl alcohol-4-b-D-gluco- side(3) and eukovoside (3,4-dihydroxy-4-phenethyl-O-a-L-rhamno- side(13)-4-O-isoferuoyl-b-D- glucoside)(4) from E. rostkoviana.
Tannins About 12%. Condensed and hydrolysable; gallic acid is among the hydrolysis products.(1)
Volatile oils About 0.2%. Seven major and numerous minor components, mainly unidentified; four of the major compounds are thought to be aldehydes or ketones.(1)
Other constituents Bitter principle, b-carotene, phytosterols (e.g. b-sitosterol, stigmasterol),(1) resin, carbohydrates (e.g. arabinose, glucose, galactose) and vitamin C.
Food Use
Eyebright is listed by the Council of Europe as a natural source of food flavouring (category N3). This category indicates that eyebright can be added to foodstuffs in the traditionally accepted manner, although there is insufficient information available for an adequate assessment of potential toxicity.(G16)
Herbal Use
Eyebright is stated to possess anticatarrhal, astringent and antiinflammatory properties. Traditionally it has been used for nasal
catarrh, sinusitis and specifically for conjunctivitis when applied locally as an eye lotion.(G2, G7, G64)
Figure 1 Selected constituents of eyebright.
256

Eyebright 257
Figure 2 Eyebright (Euphrasia officinalis).
Dosage
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Dried herb 2–4 g as an infusion three times daily.(G7)
Liquid extract 2–4 mL (1 : 1 in 25% alcohol) three times daily.(G7)
Tincture 2–6 mL (1 : 5 in 45% alcohol) three times daily.(G7)
Pharmacological Actions
In vitro and animal studies
None documented for eyebright. Caffeic acid is bacteriostatic,(1) and a purgative action in mice has been documented for iridoid glycosides.(6) The purgative action of aucubin is approximately 0.05 times the potency of sennosides, with onset of diarrhoea stated to occur more than six hours after aucubin administration.(6) Tannins are known to possess astringent properties.
Clinical studies
There is a lack of clinical research assessing the effects of eyebright and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
There is a lack of clinical safety and toxicity data for eyebright and further investigation of these aspects is required. Information in older literature states that doses of as little as 10–60 drops of eyebright tincture could lead to adverse effects, including mental confusion,(G22) although this requires confirmation.
Contra-indications, Warnings
The use of eyebright for ophthalmic application has been discouraged.(G60)
E
Figure 3 Eyebright – dried drug substance (herb).
Drug interactions None documented. However, the potential for preparations of eyebright to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered.
Pregnancy and lactation The safety of eyebright has not been established. In view of the lack of pharmacological and toxicity data, the use of eyebright during pregnancy and lactation should be avoided.
Preparations
Proprietary single-ingredient preparations
UK: Snore Calm.
Proprietary multi-ingredient preparations
Australia: Euphrasia Complex; Euphrasia Compound; Eye Health Herbal Plus Formula 4; Lifesystem Herbal Plus Formula 5 Eye Relief; Sambucus Complex. Italy: Eulux; Iridil. Malaysia: Eyebright Plus. Switzerland: Collypan; Oculosan; Tendro. UK: Se-Power; Summertime Tea Blend; Vital Eyes. USA: Eye Support Formula Herbal Blend.
References
1Harkiss KJ, Timmins P. Studies in the Scrophulariaceae Part VIII. Phytochemical investigation of Euphrasia officinalis. Planta Med 1973;
23: 342–347.
2Sticher O, Salama O. Iridoid glucosides from Euphrasia rostkoviana. Planta Med 1981; 42: 122–123.
3Salama O et al. A lignan glucoside from Euphrasia rostkoviana. Phytochemistry 1981; 20: 2003–2004.
4Sticher O et al. Structure analysis of eukovoside, a new phenylpropanoid glycoside from Euphrasia rostkoviana. Planta Med 1982; 45: 159.
5 Salama O, Sticher O. Iridoidglucoside von Euphrasia rostkoviana 4. Mitteilung über Euphrasia-Glykoside. Planta Med 1983; 47: 90–94.
6Inouye H et al. Purgative activities of iridoid glycosides. Planta Med 1974; 25: 285–288.

False Unicorn
Summary and Pharmaceutical Comment
The chemistry of false unicorn is poorly documented and no scientific evidence was located to justify the herbal uses. In view of this and the lack of toxicity data, the use of false unicorn should be avoided.
FSpecies (Family)
Chamaelirium luteum (L.) A. Gray (Melanthiaceae)
Synonym(s)
Blazing Star, Chamaelirium carolianum Wild., Devil's-bit, Helonias, Helonias dioica (Walter) Pursh., Helonias lutea (L.) Ker Gawl., Starwort, Veratrum luteum L.
Part(s) Used
Rhizome, root
Pharmacopoeial and Other Monographs
BHP 1996(G9)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General References G40 and G48.
Limited chemical information is available on false unicorn. It is stated to contain a steroidal saponin glycoside, chamaelirin, and another glycoside helonin.
Food Use
False unicorn is not used in foods.
Herbal Use
False unicorn is stated to possess an action on the uterus. Traditionally it has been used for ovarian dysmenorrhoea, leucorrhoea and specifically for amenorrhoea. It is reported to
be useful for vomiting of pregnancy and threatened miscarria-
ge.(G7, G8, G64)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Dried rhizome/root 1–2 g as an infusion three times daily.(G7)
Liquid extract 1–2 mL (1 : 1 in 45% alcohol) three times daily.(G7)
Tincture 2–5 mL (1 : 5 in 45% alcohol) three times daily.(G7)
Pharmacological Actions
None documented.
Clinical studies
There is a lack of clinical research assessing the effects of centaury and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
There is a lack of clinical safety and toxicity data for false unicorn and further investigation of these aspects is required. It is stated
Figure 1 False unicorn (Chamaelirium luteum).
Figure 2 False unicorn – dried drug substance (root).
258

that large doses of false unicorn may cause nausea and vomiting.(G7)
Contra-indications, Warnings
None documented.
Drug interactions None documented. However, in view of the lack of phytochemical and pharmacological information for false unicorn, the potential for preparations of false unicorn to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should still be considered.
False Unicorn |
259 |
Pregnancy and lactation The safety of false unicorn has not been established. In view of the lack of phytochemical, pharmacological and toxicity data, and its reputed action as a uterine tonic, the use of false unicorn during pregnancy and lactation should be avoided.
Preparations
Proprietary multi-ingredient preparations
Australia: Nervatona Plus. UK: Period Pain Relief.
F
