
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index
Damiana
Summary and Pharmaceutical Comment
There is limited chemical information available on damiana. There has been little documented evidence to justify the herbal uses, and the reputation of damiana as an aphrodisiac is unproven. In view of the lack of toxicity data and reported cyanogenetic and arbutin constituents, excessive use of damiana should be avoided.
Species (Family)
Turnera diffusa Willd. ex Schult. (Turneraceae)
Synonym(s)
Turnera aphrodisiaca Ward, T. diffusa var. aphrodisiaca (Ward) Urb.
Part(s) Used
Leaf, stem
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General Reference G6.
Carbohydrates Gum 13.5%, starch 6%, sugars.
Cyanogenetic glycosides Tetraphyllin B.(1)
Phenolic glycoside Arbutin (up to 0.7%).(2)
Tannins 3.5%. Type unspecified.
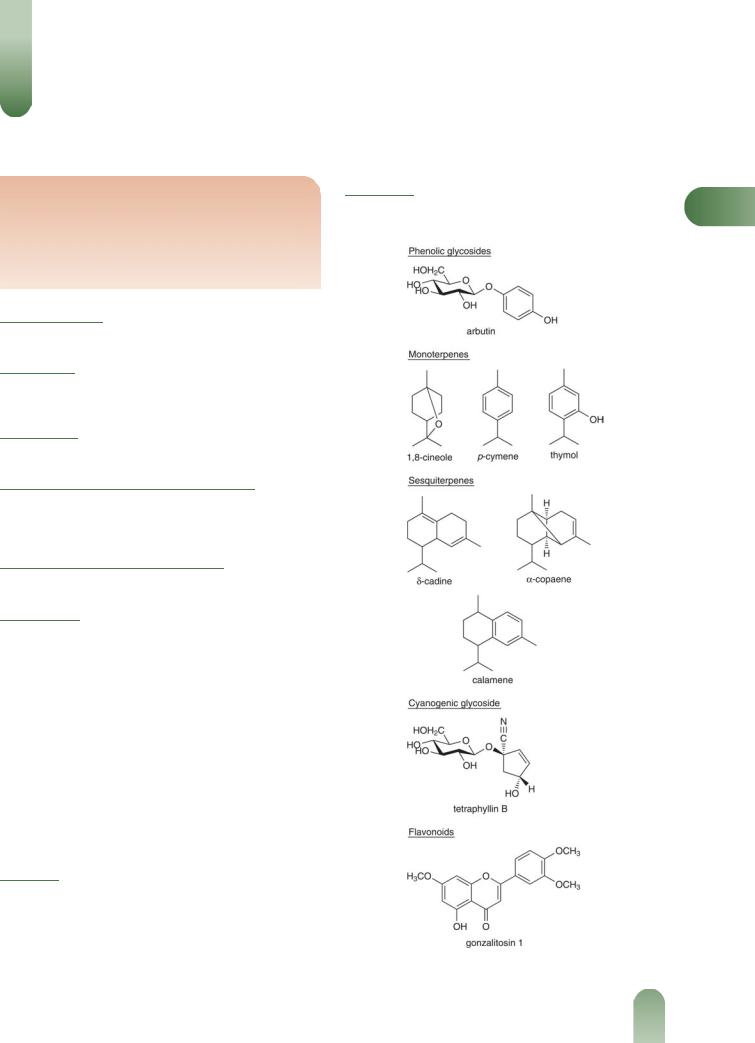
Volatile oils 0.5–1.0%. At least 20 components including 1,8- cineole (11%), p-cymene (2%), a- and b-pinene (2%), thymol, a- copaene, d-cadinene and calamene. The presence of 1,8-cineole and p-cymene has been disputed.(2)
Other constituents Acids (fatty, plant), alkanes (e.g. hexacosa- nol-1 and triacontane), damianin (7%) (a bitter principle), flavone (gonzalitosin-1), b-sitosterol, resin (6.5%).(3)
Food Use
Damiana is used in foods and is listed by the Council of Europe as a natural source of food flavouring (category N2). This category indicates that damiana can be added to foodstuffs in small
quantities with a possible limitation of an active principle (as yet unspecified) in the final product.(G16) Previously in the USA, damiana has been approved for food use.(G41)
Herbal Use
Damiana is stated to possess antidepressant, thymoleptic, mild |
D |
purgative, stomachic and reputedly aphrodisiac properties.(4) It |
Figure 1 Selected constituents of damiana.
201

202 Damiana
has been used for depression, nervous dyspepsia, atonic constipation, coital inadequacy, and specifically for anxiety neurosis with a predominant sexual factor.(G6, G7, G8, G64)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in older and contemporary standard reference texts
Dare given below.
Dried leaf 2–4 g as an infusion three times daily.(G6, G7)
Liquid Extract of Damiana (BPC 1934) 2–4 mL.
Pharmacological Actions
In vitro and animal studies
Hypoglycaemic activity has been reported in mice following both oral and intraperitoneal administration of damiana.(5) An
ethanolic extract was stated to exhibit CNS-depressant activity although no other experimental details were available.(6)
Figure 2 Damiana (Turnera diffusa).
Figure 3 Damiana – dried drug substance.
Antibacterial activity against Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus has been documented for a mixed herbal preparation, with some of the activity attributed to damiana.(7) The same herbal preparation was also reported to inhibit acetylcholine-induced spasm of the isolated guinea-pig ileum, although none of the antispasmodic activity was attributed to damiana.(7)
Arbutin is stated to be responsible for the urinary antiseptic properties (see Uva-Ursi). However, the arbutin content of damiana is much less than that quoted for uva-ursi (0.7% and 5 to 18%, respectively).
Clinical studies
There is a lack of clinical research assessing the effects of damiana and rigorous randomised controlled clinical trials are required.
A herbal preparation containing damiana as one of the ingredients was reported to have a favourable effect on the symptoms of irritable bladder associated with functional and neurohormonal disorders, and on bacterial bladder infections.(7) However, because of the methodological limitations of this study, these effects cannot be attributed to damiana.
Side-effects, Toxicity
There is a lack of clinical safety and toxicity data for damiana and further investigation of these aspects is required.
Tetanus-like convulsions and paroxysms resulting in symptoms similar to those of rabies or strychnine poisoning have been described in one individual following the ingestion of approximately 200 g damiana extract; cyanide poisoning was considered to be a possible cause. No other reported side-effects for damiana were located.
High doses of arbutin (e.g. 1 g) are considered to be toxic, although the concentration of arbutin documented for damiana (1 g arbutin is equivalent to more than 100 g plant material) is probably too low to warrant concerns over safety.
Contra-indications, Warnings
Excessive use should be avoided because of the presence of cyanogenetic glycosides and arbutin.
Drug interactions None documented. However, the potential for preparations of damiana to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered. There is limited evidence from preclinical studies that damiana has hypoglycaemic activity.
Pregnancy and lactation The safety of damiana has not been established. In view of the lack of toxicity data and possible cyanogenetic constituents, doses greatly exceeding amounts used in foods should not be taken during pregnancy or lactation.
Preparations
Proprietary multi-ingredient preparations
Australia: Bioglan Mens Super Soy/Clover; Bioglan The Blue One; Medinat Esten; Nevaton. Canada: Damiana-Sarsaparilla Formula. Italy: Dam; Four-Ton. Malaysia: Total Man. Spain: Energysor. UK: Daily Fatigue Relief; Damiana and Kola Tablets; Elixir Damiana and Saw Palmetto; Regina Royal Concorde; Strength; Strength Tablets; Supa-Tonic Tablets; VitAmour; Zotrim. USA: Women's Menopause Formula.

References
1Spencer KC, Siegler DS. Tetraphyllin B from Turnera diffusa. Planta Med 1981; 43: 175–178.
2 Auterhoff H, Häufel H-P. Inhaltsstoffe der damiana-droge. Arch Pharm 1968; 301: 537–544.
3Domínguez XA, Hinojosa M. Mexican medicinal plants. XXVIII Isolation of 5-hydroxy-7,30,40-trimethoxy-flavone from Turnera diffusa. Planta Med 1976; 30: 68–71.
Damiana 203
4 Braun JK, Malone MH. Legal highs. Clin Toxicol 1978; 12: 1–31.
5 Pérez RM et al. A study of the hypoglycemic effect of some Mexican plants. J Ethnopharmacol 1984; 12: 253–262.
6 Jiu J. A survey of some medical plants of Mexico for selected biological activity. Lloydia 1966; 29: 250–259.
7Westendorf J. Carito-In-vitro-Untersuchungen zum Nachweis spasmolytischer und kontraktiler Einflüsse. Therapiewoche 1982; 32: 6291–6297.
D
Dandelion
Summary and Pharmaceutical Comment
DDandelion is a well-known traditional herbal remedy, although limited scientific information, particularly clinical research, is available to justify the reputed uses. Several investigations have failed to demonstrate significant diuretic effects in laboratory animals and have proposed that any diuretic activity is due to the high potassium content of the leaf and root. Dandelion has also been used in foods for many years. Animal studies indicate dandelion to be of low toxicity. However, excessive ingestion of dandelion, particularly in amounts exceeding those normally consumed in foods, should be avoided.
Species (Family)
Taraxacum officinale Weber (Asteraceae/Compositae)
Synonym(s)
Lion's Tooth,Taraxacum palustre (Lyons) Lam & DC., Leontodon taraxacum L., Taraxacum
Part(s) Used
Leaf, root
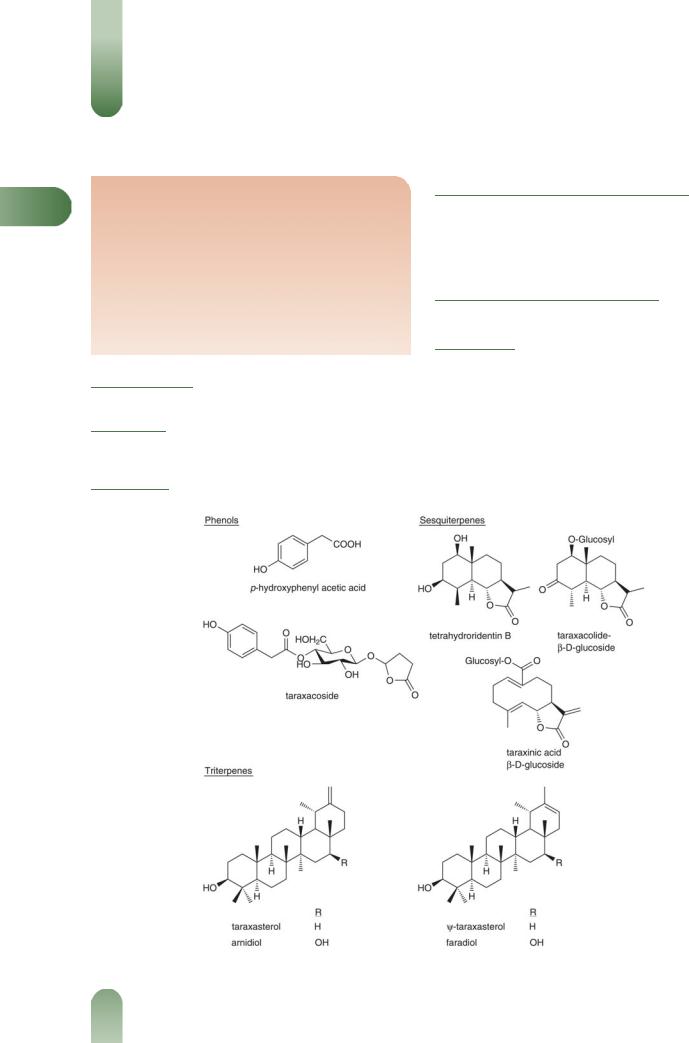
Figure 1 Selected constituents of dandelion.
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
Complete German Commission E(G3)
ESCOP 2003(G76)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General References G2, G6 and G64.
Acids and phenols Caffeic acid, p-hydroxyphenylacetic acid, chlorogenic acid,(1) cichoric acid, monocaffeoyl tartaric acids,(2) taraxacoside, linoleic acid, linolenic acid, oleic acid and palmitic acid.
Coumarins Cichoriin and aesculin.(2)
Flavonoids Luteolin-7-glucoside and luteolin-7-diglucosides.(2)
Minerals Potassium 4.5% in leaf, 2.45% in root.(3)
204

Resin Undefined bitter complex (taraxacin).
Terpenoids Sesquiterpene lactones taraxinic acid (germacranolide) esterified with glucose,(4) and eudesmanolides.(5)
Vitamins Vitamin A 14 000 iu/100 g leaf (compared with 11 000 iu/100 g carrots).
Other constituents Carotenoids, choline, inulin, pectin, phytosterols (e.g. sitosterol, stigmasterol, taraxasterol, homotaraxasterol), sugars (e.g. fructose, glucose, sucrose), triterpenes (e.g. b-amyrin, taraxol, taraxerol).
Food Use
Dandelion is used as a food, mainly in salads and soups. The roasted root and its extract have been used as a coffee substitute.(G41) Dandelion is listed by the Council of Europe as a natural source of food flavouring (category N2). This category indicates that dandelion can be added to foodstuffs in small
quantities, with a possible limitation of an active principle (as yet unspecified) in the final product.(G16) Previously in the USA,
dandelion has been listed as GRAS (Generally Recognised As Safe).(G41)
Herbal Use
Dandelion is stated to possess diuretic, laxative, cholagogue and antirheumatic properties. It has been used for cholecystitis, gallstones, jaundice, atonic dyspepsia with constipation, muscular rheumatism, oliguria, and specifically for cholecystitis and dyspepsia. The German Commission E approved use of root and
herb for disturbance of bile flow, stimulation of diuresis, loss of appetite and dyspepsia.(G3) Root is used in combination with
celandine herb and artichoke for epigastric discomfort due to functional disorders of the biliary system.(G3)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in older and contemporary standard herbal reference texts are given below.
Dried leaf 4–10 g as an infusion three times daily.(G6, G7)
Leaf, liquid extract 4–10 mL (1 : 1 in 25% alcohol) three times
daily.(G6, G7)
Leaf tincture 2–5 mL.(G3)
Dandelion 205
Leaf, fresh juice 5–10 mL.(G52)
Dried root 2–8 g or by infusion or decoction three times
daily.(G6, G7)
Root, tincture 5–10 mL (1 : 5 in 45% alcohol) three times
daily.(G6, G7)
Liquid Extract of Taraxacum (BPC 1949) 2–8 mL.
Juice of Taraxacum |
(BPC 1949) 4–8 mL. |
D |
|
Pharmacological Actions
In vitro and animal studies
A diuretic effect in rats and mice has been documented for dandelion extracts, following oral administration.(6) Herb extracts were found to produce greater diuresis than root extracts; a dose of 50 mL (equivalent to 2 g dried herb/kg body weight) produced an effect comparable to that of furosemide 80 mg/kg. By contrast, no significant increases in urine volume or sodium excretion were observed in mice following oral administration of either leaf or root extracts, or of purified fractions.(3) Similarly, oral and intravenous administration of an ethanolic extract of dandelion root failed to produce a diuretic effect in laboratory animals.(7)
Moderate anti-inflammatory activity against carrageenaninduced rat paw oedema has been documented for a dandelion root extract.(8) An 80% ethanol extract of root (100 mg/kg orally)
Figure 3 Dandelion – dried drug substance (leaf).
Figure 2 Dandelion (Taraxacum officinale). |
Figure 4 Dandelion – dried drug substance (root). |

206 Dandelion
inhibited oedema by 43% in the carrageenan-induced rat paw oedema test at 3 hours.(7)
Bile secretion was doubled in dogs by a decoction of fresh root
(equivalent to 5 g dried plant); similar activity has been observed for rats.(G52)
Hypoglycaemic activity has been described in normal, but not in diabetic rabbits, following oesophageal administration of dandelion.(9) Doses greater than 500 mg/kg produced a significant
Dblood glucose concentration which had returned to normal after 24 hours. The maximum decrease produced by a dose of 2 g/kg was reported to be 65% of the effect produced by tolbutamide 500 mg/kg. Sulfonylureas (e.g. tolbutamide) act by stimulating pancreatic beta-cells and a similar mechanism was proposed for dandelion.
In vitro antitumour activity has been documented for an
aqueous extract of dandelion, given by intraperitoneal injection, in the tumour systems ddY-Ehrlich and C3H/He-MM46.(10) The mechanism of action was thought to be similar to that of tumour polysaccharides such as lentinan.
Clinical studies
There is a lack of clinical research assessing the effects of dandelion and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
There is a lack of clinical safety and toxicity data for dandelion and further investigation of these aspects is required.
Contact allergic reactions to dandelion have been documented(11, G51) and animal studies have reported dandelion to have a
weak sensitising capacity.(12) Sesquiterpene lactones are thought to be the allergenic principles in dandelion.(4) These compounds contain an exocyclic a-methylene b-lactone moiety, which is thought to be a prerequisite for allergenic activity of sesquiterpene lactones.
The acute toxicity of dandelion appears to be low, with LD50 values (mice, intraperitoneal injection) estimated at 36.8 g/kg and 28.8 g/kg for the root and herb, respectively.(6) No visible signs of
toxicity were observed in rabbits administered dandelion 3, 4, 5 and 6 g/kg body weight by mouth for up to seven days.(9) In
addition, no behavioural changes were recorded.
Contra-indications, Warnings
Treatment with dandelion is contraindicated for patients with occlusion of bile duct, gall bladder empyema and obstructive ileus.(G3, G52) Dandelion may precipitate an allergic reaction in susceptible individuals, although no reports following the ingestion of dandelion have been documented.
Drug interactions None documented. However, the potential for preparations of dandelion to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered. There is limited evidence from preclinical studies that dandelion has diuretic and hypoglycaemic activities.
Pregnancy and lactation There are no known problems with the use of dandelion during pregnancy, provided that doses do not greatly exceed the amounts used in foods.
Preparations
Proprietary single-ingredient preparations
Czech Republic: Gallentee. Germany: Carvicum.
Proprietary multi-ingredient preparations
Argentina: Quelodin F. Australia: Berberis Complex; Bioglan Cranbiotic Super; Colax; Colax; Digest; Extralife Fluid-Care; Extralife Liva-Care; Feminine Herbal Complex; Fluid Loss; Glycoplex; Herbal Cleanse; Herbal Diuretic Formula; Lifesystem Herbal Formula 7 Liver Tonic; Liver Tonic Herbal Formula 6; Livstim; Livton Complex; Profluid; Silybum Complex; St Mary's Thistle Plus; Trifolium Complex; UvaUrsi Complex; Uva-Ursi Plus. Austria: Gallenund Lebertee St Severin; Magentee St Severin; Montana; Original Schwedenbitter; Urelium Neu. Canada: Milk Thistle Extract Formula. Czech Republic: Cynarosan; Diabetan; Diabeticka Cajova Smes-Megadiabetin; The Salvat; Ungolen. France: Diacure; Hydracur; Romarene. Germany: Alasenn; Amara-Tropfen; Carmol Magen-Galle-Darm; Cholosom SL; Cholosom-Tee; Gallemolan forte; Gallexier; Gallexier; Tonsilgon. Hong Kong: Hepatofalk. Italy: Varicofit. Malaysia: Dandelion Complex.
Russia: Tonsilgon N (Тонзилгон Н). South Africa: Amara.
Spain: Diurette. Switzerland: Boldocynara; Demonatur Gouttes pour le foie et la bile; Gastrosan; Heparfelien; Strath Gouttes pour les reins et la vessie; Tisane hepatique et biliaire. UK: Adios; Aqualette; Aqualette; Backache; Boldex; Culpeper DeTox Tea; Fenneherb Slim Aid; HealthAid Boldo-Plus; Herbulax; HRI Water Balance; Napiers Slimming Tablets; Natravene; Natural Herb Tablets; Nervous Dyspepsia Tablets; Out-of-Sorts; Reducing (Slimming) Tablets; Rheumatic Pain; Stomach Mixture; Uvacin; Uvacin; Weight Loss Aid; Wind & Dyspepsia Relief. USA: Liver Formula Herbal Blend.
References
1Clifford MN et al. The chlorogenic acids content of coffee substitutes. Food Chem 1987; 24: 99–107.
2 Williams CA et al. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochem 1996; 42: 121–127.
3 Hook I et al. Evaluation of dandelion for diuretic activity and variation in potassium content. Int J Pharmacog 1993; 31: 29–34.
4Hausen BM. Taraxinsäure-10-O-b-D-glucopyranosid, das kontaktallergen des löwenzahns (Taraxacum officinale Wiggers).
Dermatosen 1982; 30: 51–53.
5Hänsel R et al. Sequiterpenlacton-b-D-glucopyranoside sowie ein neues eudesmanolid aus Taraxacum officinale. Phytochem 1980; 19:
857–861.
6Rácz-Kotilla et al. The action of Taraxacum officinale extracts on the body weight and diuresis of laboratory animals. Planta Med 1974; 26:
212–217.
7 Tita B et al. Taraxacum officinale W.: Pharmacological effect of ethanol extract. Pharmacol Res 1993; 27: 23–24.
8Mascolo N et al. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phytother Res 1987; 1: 28–29.
9Akhtar MS et al. Effects of Portulaca oleracae (kulfa) and Taraxacum officinale (dhudhal) in normoglycaemic and alloxan-treated hyperglycaemic rabbits. J Pak Med Assoc 1985; 35: 207–210.
10Baba K et al. Antitumor activity of hot water extract of dandelion, Taraxacum officinale – correlation between antitumor activity and timing of administration. Yagugaku Zasshi 1981; 101: 538–543.
11Hausen BM, Schulz KH. Allergische kontaktdermatitis durch löwenzahn (Taraxacum officinale Wiggers). Dermatosen 1978; 26: 198.
12Davies MG, Kersey PJW. Contact allergy to yarrow and dandelion.
Contact Dermatitis 1986; 14: 256–257.
